Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography
- PMID: 26084803
- PMCID: PMC4482228
- DOI: 10.1126/scitranslmed.3010611
Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography
Abstract
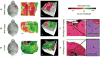
More complete brain cancer resection can prolong survival and delay recurrence. However, it is challenging to distinguish cancer from noncancer tissues intraoperatively, especially at the transitional, infiltrative zones. This is especially critical in eloquent regions (for example, speech and motor areas). This study tested the feasibility of label-free, quantitative optical coherence tomography (OCT) for differentiating cancer from noncancer in human brain tissues. Fresh ex vivo human brain tissues were obtained from 32 patients with grade II to IV brain cancer and 5 patients with noncancer brain pathologies. On the basis of volumetric OCT imaging data, pathologically confirmed brain cancer tissues (both high- and low-grade) had significantly lower optical attenuation values at both cancer core and infiltrated zones when compared with noncancer white matter, and OCT achieved high sensitivity and specificity at an attenuation threshold of 5.5 mm(-1) for brain cancer patients. We also used this attenuation threshold to confirm the intraoperative feasibility of performing in vivo OCT-guided surgery using a murine model harboring human brain cancer. Our OCT system was capable of processing and displaying a color-coded optical property map in real time at a rate of 110 to 215 frames per second, or 1.2 to 2.4 s for an 8- to 16-mm(3) tissue volume, thus providing direct visual cues for cancer versus noncancer areas. Our study demonstrates the translational and practical potential of OCT in differentiating cancer from noncancer tissue. Its intraoperative use may facilitate safe and extensive resection of infiltrative brain cancers and consequently lead to improved outcomes when compared with current clinical standards.
Copyright © 2015, American Association for the Advancement of Science.
Figures




References
-
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. - PubMed
-
- Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. - PubMed
-
- Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735–745. - PubMed
-
- McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–162. - PubMed
-
- McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65:463–469. discussion 469-470. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

