The Hand-Foot Skin Reaction and Quality of Life Questionnaire: An Assessment Tool for Oncology
- PMID: 26084809
- PMCID: PMC4492225
- DOI: 10.1634/theoncologist.2014-0219
The Hand-Foot Skin Reaction and Quality of Life Questionnaire: An Assessment Tool for Oncology
Abstract
Background: Skin toxicity (hand-foot syndrome/hand-foot skin reaction, HFS/R) related to antineoplastic therapy is a significant issue in oncology practice, with potentially large impacts on health-related quality of life (HRQL).
Materials and methods: A patient-reported questionnaire, the hand-foot skin reaction and quality of life (HF-QoL) questionnaire was developed to measure the HFS/R symptoms associated with cancer therapeutic agents and their effect on daily activities. The validity and reliability of the HF-QoL questionnaire was tested in a randomized trial of capecitabine with sorafenib/placebo in 223 patients with locally advanced/metastatic breast cancer. Other measures completed included patient ratings of condition severity, the Functional Assessment of Cancer Therapy-Breast cancer (FACT-B), and the clinician-rated National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0, hand-foot skin reaction grade. The psychometric properties of the HF-QoL tested included structural validity, internal consistency, construct validity, discriminant validity, and responsiveness. Finally, the minimal clinically important difference (MCID) was estimated.
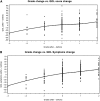
Results: The HF-QoL instrument comprises a 20-item symptom scale and an 18-item daily activity scale. Each scale demonstrated excellent measurement properties and discriminated between NCI-CTCAE grade and patient-rated condition severity with large effect sizes. The daily activity scale had excellent internal consistency and correlated with the FACT-B and HF-QoL symptom scores. Both HF-QoL scale scores increased linearly with increasing patient-rated condition severity. The MCIDs were estimated as 5 units for daily activities and 8 units for symptoms mean scores.
Conclusion: The HF-QoL was sensitive to symptoms and HRQL issues associated with HFS/R among participants treated with capecitabine with and without sorafenib. The HF-QoL appears suitable for assessing the HRQL impairment associated with HFS/R to cancer therapies.
Implications for practice: Skin toxicity related to anticancer therapies is a significant issue in oncology practice. Several newer agents, as well as older therapies, are associated with the skin toxicity known as hand-foot skin reaction (HFSR) or hand-foot syndrome (HFS). This study describes the development and validation of a brief, patient-reported questionnaire (the hand-foot skin reaction and quality of life questionnaire) supporting its suitability for use in clinical research to aid in early recognition of symptoms, to evaluate the effectiveness of agents for HFS/R treatment within clinical trials, and to evaluate the impact of these treatments on HFS/R-associated patients' health-related quality of life.
Keywords: Hand-foot skin reaction; Hand-foot syndrome; Multikinase inhibitors; Oncology; Quality of life; Symptom assessment.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Anderson R, Jatoi A, Robert C, et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs) The Oncologist. 2009;14:291–302. - PubMed
-
- Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. - PubMed
-
- Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (“hand-foot”) syndrome: Incidence, recognition and management. Am J Clin Dermatol. 2000;1:225–234. - PubMed
-
- Scheithauer W, Blum J. Coming to grips with hand-foot syndrome: Insights from clinical trials evaluating capecitabine. Oncology (Williston Park) 2004;18:1161–1168, 1173, discussion 1173–1176, 1181–1184. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

