Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome
- PMID: 26084842
- PMCID: PMC4551345
- DOI: 10.15252/emmm.201404929
Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome
Abstract
Mutations in the potassium channel subunit KCNQ1 cause the human severe congenital deafness Jervell and Lange-Nielsen (JLN) syndrome. We applied a gene therapy approach in a mouse model of JLN syndrome (Kcnq1(-/-) mice) to prevent the development of deafness in the adult stage. A modified adeno-associated virus construct carrying a Kcnq1 expression cassette was injected postnatally (P0-P2) into the endolymph, which resulted in Kcnq1 expression in most cochlear marginal cells where native Kcnq1 is exclusively expressed. We also found that extensive ectopic virally mediated Kcnq1 transgene expression did not affect normal cochlear functions. Examination of cochlear morphology showed that the collapse of the Reissner's membrane and degeneration of hair cells (HCs) and cells in the spiral ganglia were corrected in Kcnq1(-/-) mice. Electrophysiological tests showed normal endocochlear potential in treated ears. In addition, auditory brainstem responses showed significant hearing preservation in the injected ears, ranging from 20 dB improvement to complete correction of the deafness phenotype. Our results demonstrate the first successful gene therapy treatment for gene defects specifically affecting the function of the stria vascularis, which is a major site affected by genetic mutations in inherited hearing loss.
Keywords: Jervell and Lange‐Nielsen syndrome; Kcnq1 null mice; gene therapy; hearing restoration; virus.
© 2015 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

A Diagram showing the major landmarks of the cochlear section to facilitate comparison of data shown in (B–F).
B–F Immunolabeling results of Kcnq1 (green) in cochlear cryosections are shown for uninjected WT (B), untreated Kcnq1−/− (C), injected WT (D), and Kcnq1−/− mice given injections into the ST (E) or SM (F). Cell nuclei were outlined by counterstaining with either DAPI (B–D, F) or Qnuclear deep red (E). Scale bars represent approximately 100 μm. Meaning of white arrows are given in the text.
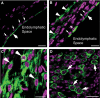
Cryosection through the SV of WT mice. The large arrow points to the native Kcnq1 (labeled green) in WT mice. Smaller arrows show nuclei of the marginal cells.
Cryosection through the SV of a Kcnq1−/− mouse injected with AAV expressing Kcnq1. Labeled in green (bigger arrow) is the AAV1-expressed Kcnq1, found only in the apical membrane of the marginal cells. Smaller arrows show nuclei of the marginal cells. Arrowheads show Kcnq1 immunolabeling in fibrocytes outside the SV.
Cryosection through the lateral wall of Kcnq1−/− mice showing intracellular distribution of Kcnq1 immunolabeling (green) in fibrocytes.
Cryosection through the spiral ganglia of cochlea of treated Kcnq1−/− mice showing immunolabeling (green) in the cells of spiral ganglia (arrows).
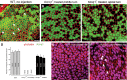
A Immunolabeling results (Kcnq1 labeled in green) in WT mice.
B, C Immunolabeling results (Kcnq1 labeled in green) of treated Kcnq1−/− mice, middle (B) and apical (C) turns, respectively.
D The percentage of marginal cells having positive Kcnq1 immunolabeling signal above a visually detectable level is shown for WT (gray bars, left), untreated Kcnq1−/− (middle), and treated Kcnq1−/− mice (black bars, right). Data are given as mean ± SD (n = 6).
E, F The organization of marginal cells in the SV is outlined by labeling with phalloidin conjugated with rhodamine. Results from WT (E) and untreated Kcnq1−/− mice (F) are compared.

A–C Cochlear sections obtained from WT (A), nontreated Kcnq1−/− (B), and treated Kcnq1−/− mice (C) were compared. Major landmarks of the cochlear sections are labeled and pointed by arrows. Scale bars represent approximately 50 μm.
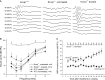
Waveforms of ABRs are compared in WT, untreated Kcnq1−/−, and treated Kcnq1−/− mice, as labeled above data traces. Series of averaged ABR data traces were evoked from tone-burst sounds with intensities ranging from 20 to 90 dB SPL.
Summary of averaged ABR thresholds at various frequencies for different groups of mice; untreated Kcnq1−/− (open triangles), treated Kcnq1−/− (filled triangles), injected WT (filled circles), and uninjected WT (filled squares). Plot legends are given in the figure. The plot with open circles connected with dashed lines represents average data from the five best cases of treated Kcnq1−/− mice. Error bars represent standard error of the mean.
Click-evoked ABR thresholds of WT (filled squares), treated Kcnq1−/− mice (filled triangles), untreated Kcnq1−/− mice (open squares) measured 4–30 weeks after mice were born. Error bars represent standard error of the mean. Upward arrows indicate that click-ABR thresholds were at the maximal sound level that could be reliably measured by the system.
References
-
- Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A, Ehrhardt A, et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods. 2012;23:18–28. - PubMed
-
- Avraham KB, Kanaan M. Genomic advances for gene discovery in hereditary hearing loss. J Basic Clin Physiol Pharmacol. 2012;23:93–97. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

