Neuronal variability in orbitofrontal cortex during economic decisions
- PMID: 26084903
- PMCID: PMC4556853
- DOI: 10.1152/jn.00231.2015
Neuronal variability in orbitofrontal cortex during economic decisions
Abstract
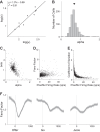
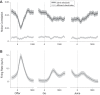
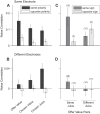
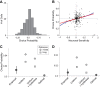
Neuroeconomic models assume that economic decisions are based on the activity of offer value cells in the orbitofrontal cortex (OFC), but testing this assertion has proven difficult. In principle, the decision made on a given trial should correlate with the stochastic fluctuations of these cells. However, this correlation, measured as a choice probability (CP), is small. Importantly, a neuron's CP reflects not only its individual contribution to the decision (termed readout weight), but also the intensity and the structure of correlated variability across the neuronal population (termed noise correlation). A precise mathematical relation between CPs, noise correlations, and readout weights was recently derived by Haefner and colleagues (Haefner RM, Gerwinn S, Macke JH, Bethge M. Nat Neurosci 16: 235-242, 2013) for a linear decision model. In this framework, concurrent measurements of noise correlations and CPs can provide quantitative information on how a population of cells contributes to a decision. Here we examined neuronal variability in the OFC of rhesus monkeys during economic decisions. Noise correlations had similar structure but considerably lower strength compared with those typically measured in sensory areas during perceptual decisions. In contrast, variability in the activity of individual cells was high and comparable to that recorded in other cortical regions. Simulation analyses based on Haefner's equation showed that noise correlations measured in the OFC combined with a plausible readout of offer value cells reproduced the experimental measures of CPs. In other words, the results obtained for noise correlations and those obtained for CPs taken together support the hypothesis that economic decisions are primarily based on the activity of offer value cells.
Keywords: neoroeconomics; subjective value; value-based decision.
Copyright © 2015 the American Physiological Society.
Figures



References
-
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput 11: 91–101, 1999. - PubMed
-
- Ahn S, Fessler JA. Globally convergent image reconstruction for emission tomography using relaxed ordered subsets algorithms. IEEE Trans Med Imaging 22: 613–626, 2003. - PubMed
-
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput 8: 1185–1202, 1996. - PubMed
-
- Britten KH, Newsome WT, Saunders RC. Effects of inferotemporal cortex lesions on form-from-motion discrimination in monkeys. Exp Brain Res 88: 292–302, 1992. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

