Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS
- PMID: 26084905
- PMCID: PMC4533062
- DOI: 10.1152/jn.00259.2015
Fidelity of frequency and phase entrainment of circuit-level spike activity during DBS
Abstract
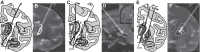
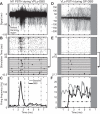
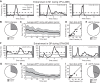
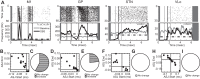
High-frequency stimulation is known to entrain spike activity downstream and upstream of several clinical deep brain stimulation (DBS) targets, including the cerebellar-receiving area of thalamus (VPLo), subthalamic nucleus (STN), and globus pallidus (GP). Less understood are the fidelity of entrainment to each stimulus pulse, whether entrainment patterns are stationary over time, and how responses differ among DBS targets. In this study, three rhesus macaques were implanted with a single DBS lead in VPLo, STN, or GP. Single-unit spike activity was recorded in the resting state in motor cortex during VPLo DBS, in GP during STN DBS, and in STN and pallidal-receiving area of motor thalamus (VLo) during GP DBS. VPLo DBS induced time-locked spike activity in 25% (n = 15/61) of motor cortex cells, with entrained cells following 7.5 ± 7.4% of delivered pulses. STN DBS entrained spike activity in 26% (n = 8/27) of GP cells, which yielded time-locked spike activity for 8.7 ± 8.4% of stimulus pulses. GP DBS entrained 67% (n = 14/21) of STN cells and 32% (n = 19/59) of VLo cells, which showed a higher fraction of pulses effectively inhibiting spike activity (82.0 ± 9.6% and 86.1 ± 16.6%, respectively). Latency of phase-locked spike activity increased over time in motor cortex (58%, VPLo DBS) and to a lesser extent in GP (25%, STN DBS). In contrast, the initial inhibitory phase observed in VLo and STN during GP DBS remained stable following stimulation onset. Together, these data suggest that circuit-level entrainment is low-pass filtered during high-frequency stimulation, most notably for glutamatergic pathways. Moreover, phase entrainment is not stationary or consistent at the circuit level for all DBS targets.
Keywords: deep brain stimulation; entrainment; globus pallidus; mechanisms; motor cortex; peri-stimulus time histogram; subthalamic nucleus; thalamus.
Copyright © 2015 the American Physiological Society.
Figures

References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. - PubMed
-
- Anderson M, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150–1160, 2003. - PubMed
-
- Bellinger SC, Miyazawa G, Steinmetz PN. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Eng 5: 263–274, 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

