AT1R blockade in adverse milieus: role of SMRT and corepressor complexes
- PMID: 26084932
- PMCID: PMC4525095
- DOI: 10.1152/ajprenal.00476.2014
AT1R blockade in adverse milieus: role of SMRT and corepressor complexes
Abstract
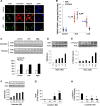
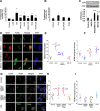
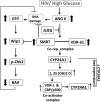
ANG II type 1 receptor blockade (AT1R-BLK) is used extensively to slow down the progression of proteinuric kidney diseases. We hypothesized that AT1R-BLK provides podocyte protection through regulation of silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) and vitamin D receptor (VDR) expression under adverse milieus such as high glucose and human immunodeficiency virus infection. Both AT1R-BLK and VDR agonists (VDAs) stimulated VDR complex formation that differed not only in their composition but also in their functionality. AT1R-BLK-induced VDR complexes contained predominantly unliganded VDR, SMRT, and phosphorylated histone deacetylase 3, whereas VDA-VDR complexes were constituted by liganded VDR and CREB-binding protein/p300. AT1R-BLK-induced complexes attenuated podocyte acetyl-histone 3 levels as well as cytochrome P-450 family 24A1 expression, thus indicating their deacetylating and repressive properties. On the other hand, VDA-VDR complexes not only increased podocyte acetyl-histone 3 levels but also enhanced cytochrome P-450 family 24A1 expression, thus suggesting their acetylating and gene activation properties. AT1R-BLK- induced podocyte SMRT inhibited expression of the proapoptotic gene BAX through downregulation of Wip1 and phosphorylation of checkpoint kinase 2 in high-glucose milieu. Since SMRT-depleted podocytes lacked AT1R-BLK-mediated protection against DNA damage, it appears that SMRT is necessary for DNA repairs during AT1R-BLK. We conclude that AT1R-BLK provides podocyte protection in adverse milieus predominantly through SMRT expression and partly through unliganded VDR expression in 1,25(OH)2D-deficient states; on the other hand, AT1R-BLK contributes to liganded VDR expression in 1,25(OH)2D-sufficient states.
Keywords: angiotensin II; angiotensin II type 1 receptor blockade; glucose; silencing mediator of retinoic acid and thyroid hormone receptor; vitamin D receptor.
Copyright © 2015 the American Physiological Society.
Figures





References
-
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev 81: 1269–1304, 2001. - PubMed
-
- Cantorna MT, Waddell A. The vitamin D receptor turns off chronically activated T cells. Ann NY Acad Sci 1317: 70–75, 2014. - PubMed
-
- Carlberg C, Mathiasen IS, Saurat JH, Binderup L. The 1,25-dihydroxyvitamin D3 (VD) analogues MC903, EB1089 and KH1060 activate the VD receptor: homodimers show higher ligand sensitivity than heterodimers with retinoid X receptors. J Steroid Biochem Mol Biol 51: 137–142, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

