Ziprasidone Augmentation of Escitalopram for Major Depressive Disorder: Efficacy Results From a Randomized, Double-Blind, Placebo-Controlled Study
- PMID: 26085041
- PMCID: PMC4843798
- DOI: 10.1176/appi.ajp.2015.14101251
Ziprasidone Augmentation of Escitalopram for Major Depressive Disorder: Efficacy Results From a Randomized, Double-Blind, Placebo-Controlled Study
Abstract
Objective: The authors sought to test the efficacy of adjunctive ziprasidone in adults with nonpsychotic unipolar major depression experiencing persistent symptoms after 8 weeks of open-label treatment with escitalopram.
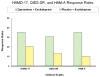
Method: This was an 8-week, randomized, double-blind, parallel-group, placebo-controlled trial conducted at three academic medical centers. Participants were 139 outpatients with persistent symptoms of major depression after an 8-week open-label trial of escitalopram (phase 1), randomly assigned in a 1:1 ratio to receive adjunctive ziprasidone (escitalopram plus ziprasidone, N=71) or adjunctive placebo (escitalopram plus placebo, N=68), with 8 weekly follow-up assessments. The primary outcome measure was clinical response, defined as a reduction of at least 50% in score on the 17-item Hamilton Depression Rating Scale (HAM-D). The Hamilton Anxiety Rating scale (HAM-A) and Visual Analog Scale for Pain were defined a priori as key secondary outcome measures.
Results: Rates of clinical response (35.2% compared with 20.5%) and mean improvement in HAM-D total scores (-6.4 [SD=6.4] compared with -3.3 [SD=6.2]) were significantly greater for the escitalopram plus ziprasidone group. Several secondary measures of antidepressant efficacy also favored adjunctive ziprasidone. The escitalopram plus ziprasidone group also showed significantly greater improvement on HAM-A score but not on Visual Analog Scale for Pain score. Ten (14%) patients in the escitalopram plus ziprasidone group discontinued treatment because of intolerance, compared with none in the escitalopram plus placebo group.
Conclusions: Ziprasidone as an adjunct to escitalopram demonstrated antidepressant efficacy in adult patients with major depressive disorder experiencing persistent symptoms after 8 weeks of open-label treatment with escitalopram.
Trial registration: ClinicalTrials.gov NCT00633399.
Figures
Comment in
-
Adjunctive Ziprasidone in Major Depression and the Current Status of Adjunctive Atypical Antipsychotics.Am J Psychiatry. 2015 Dec;172(12):1176-8. doi: 10.1176/appi.ajp.2015.15091220. Am J Psychiatry. 2015. PMID: 26619770 No abstract available.
-
ADDENDUM.Am J Psychiatry. 2015 Dec;172(12):1256. doi: 10.1176/appi.ajp.2015.17212Addendum. Am J Psychiatry. 2015. PMID: 26624958 No abstract available.
References
-
- Papakostas GI, Fava M. Monoamine-based Pharmacotherapy. In: Licinio J, Wong ML, editors. Biology of Depression: From Novel Insights to Therapeutic Strategies. 1. Weinheim: Wiley-VCH Verlag; 2005. pp. 87–140.
-
- Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009 Jan;19(1):34–40. - PubMed
-
- Papakostas GI. Managing partial response or nonresponse: switching, augmentation, and combination strategies for major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 6):16–25. - PubMed
-
- Shelton RC, Papakostas GI. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand. 2008;117(4):253–9. - PubMed
-
- Papakostas GI. Augmentation strategies in the treatment of major depressive disorder. Examining the evidence on augmentation with atypical antipsychotics. CNS Spectr. 2007;12(12 Suppl 22):10–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical