Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1
- PMID: 26085162
- PMCID: PMC4524080
- DOI: 10.1128/JVI.01232-15
Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1
Abstract
Accumulating evidence indicates a role for Fc receptor (FcR)-mediated effector functions of antibodies, including antibody-dependent cell-mediated cytotoxicity (ADCC), in prevention of human immunodeficiency virus type 1 (HIV-1) acquisition and in postinfection control of viremia. Consequently, an understanding of the molecular basis for Env epitopes that constitute effective ADCC targets is of fundamental interest for humoral anti-HIV-1 immunity and for HIV-1 vaccine design. A substantial portion of FcR effector function of potentially protective anti-HIV-1 antibodies is directed toward nonneutralizing, transitional, CD4-inducible (CD4i) epitopes associated with the gp41-reactive region of gp120 (cluster A epitopes). Our previous studies defined the A32-like epitope within the cluster A region and mapped it to the highly conserved and mobile layers 1 and 2 of the gp120 inner domain within the C1-C2 regions of gp120. Here, we elucidate additional cluster A epitope structures, including an A32-like epitope, recognized by human monoclonal antibody (MAb) N60-i3, and a hybrid A32-C11-like epitope, recognized by rhesus macaque MAb JR4. These studies define for the first time a hybrid A32-C11-like epitope and map it to elements of both the A32-like subregion and the seven-layered β-sheet of the gp41-interactive region of gp120. These studies provide additional evidence that effective antibody-dependent effector function in the cluster A region depends on precise epitope targeting--a combination of epitope footprint and mode of antibody attachment. All together these findings help further an understanding of how cluster A epitopes are targeted by humoral responses.
Importance: HIV/AIDS has claimed the lives of over 30 million people. Although antiretroviral drugs can control viral replication, no vaccine has yet been developed to prevent the spread of the disease. Studies of natural HIV-1 infection, simian immunodeficiency virus (SIV)- or simian-human immunodeficiency virus (SHIV)-infected nonhuman primates (NHPs), and HIV-1-infected humanized mouse models, passive transfer studies in infants born to HIV-infected mothers, and the RV144 clinical trial have linked FcR-mediated effector functions of anti-HIV-1 antibodies with postinfection control of viremia and/or blocking viral acquisition. With this report we provide additional definition of the molecular determinants for Env antigen engagement which lead to effective antibody-dependent effector function directed to the nonneutralizing CD4-dependent epitopes in the gp41-reactive region of gp120. These findings have important implications for the development of an effective HIV-1 vaccine.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




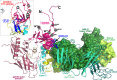
References
-
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 75:8340–8347. doi:10.1128/JVI.75.17.8340-8347.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 AI-084830/AI/NIAID NIH HHS/United States
- R01 AI087181/AI/NIAID NIH HHS/United States
- R01 AI110259/AI/NIAID NIH HHS/United States
- P41RR001209/RR/NCRR NIH HHS/United States
- R01 AI-087181/AI/NIAID NIH HHS/United States
- P41 RR001209/RR/NCRR NIH HHS/United States
- Intramural NIH HHS/United States
- K23 AI084580/AI/NIAID NIH HHS/United States
- K25-AI087968/AI/NIAID NIH HHS/United States
- I01 BX002358/BX/BLRD VA/United States
- R01 AI084830/AI/NIAID NIH HHS/United States
- 5K23AI084580-04/AI/NIAID NIH HHS/United States
- K25 AI087968/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

