A novel multiple-stage antimalarial agent that inhibits protein synthesis
- PMID: 26085270
- PMCID: PMC4700930
- DOI: 10.1038/nature14451
A novel multiple-stage antimalarial agent that inhibits protein synthesis
Erratum in
-
Corrigendum: A novel multiple-stage antimalarial agent that inhibits protein synthesis.Nature. 2016 Sep 1;537(7618):122. doi: 10.1038/nature18280. Epub 2016 May 25. Nature. 2016. PMID: 27281209 No abstract available.
Abstract
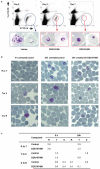
There is an urgent need for new drugs to treat malaria, with broad therapeutic potential and novel modes of action, to widen the scope of treatment and to overcome emerging drug resistance. Here we describe the discovery of DDD107498, a compound with a potent and novel spectrum of antimalarial activity against multiple life-cycle stages of the Plasmodium parasite, with good pharmacokinetic properties and an acceptable safety profile. DDD107498 demonstrates potential to address a variety of clinical needs, including single-dose treatment, transmission blocking and chemoprotection. DDD107498 was developed from a screening programme against blood-stage malaria parasites; its molecular target has been identified as translation elongation factor 2 (eEF2), which is responsible for the GTP-dependent translocation of the ribosome along messenger RNA, and is essential for protein synthesis. This discovery of eEF2 as a viable antimalarial drug target opens up new possibilities for drug discovery.
Figures









Comment in
-
Malaria: Hitting all stages of the parasite life cycle.Nat Rev Microbiol. 2015 Aug;13(8):458. doi: 10.1038/nrmicro3530. Nat Rev Microbiol. 2015. PMID: 26179353 No abstract available.
-
Malaria: Hitting all stages of the parasite life cycle.Nat Rev Drug Discov. 2015 Aug;14(8):527. doi: 10.1038/nrd4689. Epub 2015 Jul 24. Nat Rev Drug Discov. 2015. PMID: 26205463 No abstract available.
References
-
- WHO World Malaria Report 2014. 2014 ISBN 978 992 974 156483 156480.
-
- Wells TNC, Gutteridge WE. Neglected Diseases and Drug Discovery. 2012:1–32. RSC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

