Activating positive memory engrams suppresses depression-like behaviour
- PMID: 26085274
- PMCID: PMC5583720
- DOI: 10.1038/nature14514
Activating positive memory engrams suppresses depression-like behaviour
Abstract
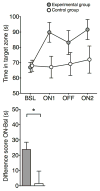
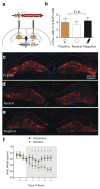
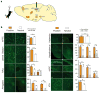
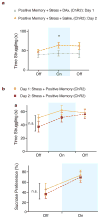
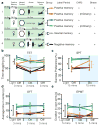
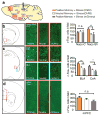
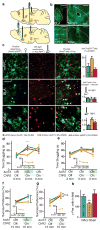
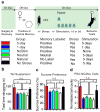
Stress is considered a potent environmental risk factor for many behavioural abnormalities, including anxiety and mood disorders. Animal models can exhibit limited but quantifiable behavioural impairments resulting from chronic stress, including deficits in motivation, abnormal responses to behavioural challenges, and anhedonia. The hippocampus is thought to negatively regulate the stress response and to mediate various cognitive and mnemonic aspects of stress-induced impairments, although the neuronal underpinnings sufficient to support behavioural improvements are largely unknown. Here we acutely rescue stress-induced depression-related behaviours in mice by optogenetically reactivating dentate gyrus cells that were previously active during a positive experience. A brain-wide histological investigation, coupled with pharmacological and projection-specific optogenetic blockade experiments, identified glutamatergic activity in the hippocampus-amygdala-nucleus-accumbens pathway as a candidate circuit supporting the acute rescue. Finally, chronically reactivating hippocampal cells associated with a positive memory resulted in the rescue of stress-induced behavioural impairments and neurogenesis at time points beyond the light stimulation. Together, our data suggest that activating positive memories artificially is sufficient to suppress depression-like behaviours and point to dentate gyrus engram cells as potential therapeutic nodes for intervening with maladaptive behavioural states.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
Neuroscience: The power of positivity.Nature. 2015 Jun 18;522(7556):294-5. doi: 10.1038/522294a. Nature. 2015. PMID: 26085266 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

