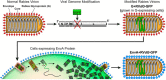
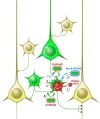
Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses
- PMID: 26085623
- PMCID: PMC4469731
- DOI: 10.1523/JNEUROSCI.0409-15.2015
Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
