Synthesis and characterization of [Zn(acetate)2(amine)x] compounds (x = 1 or 2) and their use as precursors to ZnO
- PMID: 26085801
- PMCID: PMC4465802
- DOI: 10.1016/j.mssp.2015.04.001
Synthesis and characterization of [Zn(acetate)2(amine)x] compounds (x = 1 or 2) and their use as precursors to ZnO
Abstract
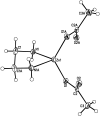
As an obvious candidate for a p-type dopant in ZnO, nitrogen remains elusive in this role. Nitrogen containing precursors are a potential means to incorporate nitrogen during MOCVD growth. One class of nitrogen-containing precursors are zinc acetate amines, yet, they have received little attention. The synthesis and single crystal X-ray structure of [Zn(acetate)2(en)], and the synthesis of [Zn(acetate)2(en)2], [Zn(acetate)2(benzylamine)2], [Zn(acetate)2(butylamine)2], [Zn(acetate)2(NH3)2], and [Zn(acetate)2(tris)2], where en = ethylenediamine and tris = (tris[hydroxymethyl]aminomethane) are reported. The compounds were characterized by thermogravimetric analysis and pyrolyzed in air and inert gas to yield ZnO. These compounds are useful single source precursors to ZnO bulk powders by alkali precipitation and ZnO thin films by spray pyrolysis. The amine bound to the zinc influences the ZnO crystal size and shape and acts as a nitrogen donor for preparing nitrogen-doped ZnO during alkali precipitation. Thin films of ZnO prepared by spray pyrolysis using the precursors had a (100) preferred orientation and measured n-type to intrinsic conductivity.
Keywords: ZnO; crystal structure; single source precursors.
Figures











References
-
- Ennaoui A, Weber M, Scheer R, Lewerenz HJ. Sol Energy Mater. Sol Cells. 1998;54:277–286.
-
- Lee JB, Lee HJ, Seo SH, Park JS. Thin Solid Films. 2001;398-399:641–646.
-
- Paraguay DF, Miki-Yoshida M, Morales J, Solis J, Estrada LW. Thin Solid Films. 2000;373:137–140.
-
- Das R, Ray S. J Phys D Appl Phys. 2003;36:152–155.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
