Host-Mediated Bioactivation of Pyrazinamide: Implications for Efficacy, Resistance, and Therapeutic Alternatives
- PMID: 26086040
- PMCID: PMC4467917
- DOI: 10.1021/id500028m
Host-Mediated Bioactivation of Pyrazinamide: Implications for Efficacy, Resistance, and Therapeutic Alternatives
Abstract
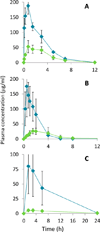
Pyrazinamide has played a critical role in shortening therapy against drug-sensitive, drug-resistant, active, and latent tuberculosis (TB). Despite widespread recognition of its therapeutic importance, the sterilizing properties of this 60-year-old drug remain an enigma given its rather poor activity in vitro. Here we revisit longstanding paradigms and offer pharmacokinetic explanations for the apparent disconnect between in vitro activity and clinical impact. We show substantial host-mediated conversion of prodrug pyrazinamide (PZA) to the active form, pyrazinoic acid (POA), in TB patients and in animal models. We demonstrate favorable penetration of this pool of circulating POA from plasma into lung tissue and granulomas, where the pathogen resides. In standardized growth inhibition experiments, we show that POA exhibits superior in vitro potency compared to PZA, indicating that the vascular supply of host-derived POA may contribute to the in vivo efficacy of PZA, thereby reducing the apparent discrepancy between in vitro and in vivo activity. However, the results also raise the possibility that subinhibitory concentrations of POA generated by the host could fuel the emergence of resistance to both PZA and POA. In contrast to widespread expectations, we demonstrate good oral bioavailability and exposure in preclinical species in pharmacokinetic studies of oral POA. Baseline exposure of oral POA can be further increased by the xanthine oxidase inhibitor and approved gout drug allopurinol. These promising results pave the way for clinical investigations of oral POA as a therapeutic alternative or an add-on to overcome PZA resistance and salvage this essential TB drug.
Keywords: Mycobacterium tuberculosis; bioactivation; host metabolism; pyrazinamide; pyrazinoic acid.
Figures




References
-
- Anonymous. Controlled trial of four thrice-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Lancet. 1981;1:171–174. - PubMed
-
- Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 8-month) for pulmonary tuberculosis: final report. East and Central African/British Medical Research Council Fifth Collaborative Study. Tubercle. 1986;67:5–15. - PubMed
-
- Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity, and acceptability. The report of final results. Ann. Int. Med. 1990;112:397–406. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
