Increased Expression of Simple Ganglioside Species GM2 and GM3 Detected by MALDI Imaging Mass Spectrometry in a Combined Rat Model of Aβ Toxicity and Stroke
- PMID: 26086081
- PMCID: PMC4473074
- DOI: 10.1371/journal.pone.0130364
Increased Expression of Simple Ganglioside Species GM2 and GM3 Detected by MALDI Imaging Mass Spectrometry in a Combined Rat Model of Aβ Toxicity and Stroke
Abstract
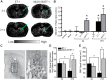
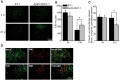
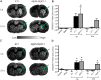
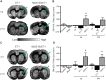
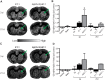
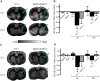
The aging brain is often characterized by the presence of multiple comorbidities resulting in synergistic damaging effects in the brain as demonstrated through the interaction of Alzheimer's disease (AD) and stroke. Gangliosides, a family of membrane lipids enriched in the central nervous system, may have a mechanistic role in mediating the brain's response to injury as their expression is altered in a number of disease and injury states. Matrix-Assisted Laser Desorption Ionization (MALDI) Imaging Mass Spectrometry (IMS) was used to study the expression of A-series ganglioside species GD1a, GM1, GM2, and GM3 to determine alteration of their expression profiles in the presence of beta-amyloid (Aβ) toxicity in addition to ischemic injury. To model a stroke, rats received a unilateral striatal injection of endothelin-1 (ET-1) (stroke alone group). To model Aβ toxicity, rats received intracerebralventricular (i.c.v.) injections of the toxic 25-35 fragment of the Aβ peptide (Aβ alone group). To model the combination of Aβ toxicity with stroke, rats received both the unilateral ET-1 injection and the bilateral icv injections of Aβ25-35 (combined Aβ/ET-1 group). By 3 d, a significant increase in the simple ganglioside species GM2 was observed in the ischemic brain region of rats who received a stroke (ET-1), with or without Aβ. By 21 d, GM2 levels only remained elevated in the combined Aβ/ET-1 group. GM3 levels however demonstrated a different pattern of expression. By 3 d GM3 was elevated in the ischemic brain region only in the combined Aβ/ET-1 group. By 21 d, GM3 was elevated in the ischemic brain region in both stroke alone and Aβ/ET-1 groups. Overall, results indicate that the accumulation of simple ganglioside species GM2 and GM3 may be indicative of a mechanism of interaction between AD and stroke.
Conflict of interest statement
Figures
References
-
- Jin YP, Østbye T, Feightner JW, Di Legge S, Hachinski V. Joint effect of stroke and APOE 4 on dementia risk: The canadian study of health and aging. Neurology. 2008;70: 9–16. - PubMed
-
- Johnston JM, Nazar-Stewart V, Kelsey SF, M IK, Ganguli M. Relationships between cerebrovascular events, APOE polymorphism and Alzheimer's disease in a community sample. Neuroepidemiology. 2000;9: 320–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

