Targeting Human Transmission Biology for Malaria Elimination
- PMID: 26086192
- PMCID: PMC4472755
- DOI: 10.1371/journal.ppat.1004871
Targeting Human Transmission Biology for Malaria Elimination
Abstract
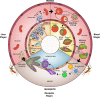
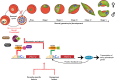
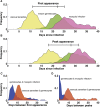
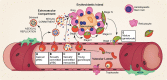
Malaria remains one of the leading causes of death worldwide, despite decades of public health efforts. The recent commitment by many endemic countries to eliminate malaria marks a shift away from programs aimed at controlling disease burden towards one that emphasizes reducing transmission of the most virulent human malaria parasite, Plasmodium falciparum. Gametocytes, the only developmental stage of malaria parasites able to infect mosquitoes, have remained understudied, as they occur in low numbers, do not cause disease, and are difficult to detect in vivo by conventional methods. Here, we review the transmission biology of P. falciparum gametocytes, featuring important recent discoveries of genes affecting parasite commitment to gametocyte formation, microvesicles enabling parasites to communicate with each other, and the anatomical site where immature gametocytes develop. We propose potential parasite targets for future intervention and highlight remaining knowledge gaps.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO (2014) WHO Malaria Report 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/
-
- Schneider P, Bousema T, Omar S, Gouagna L, Sawa P, et al. (2006) (Sub)microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol 36: 403–408. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

