SGLT2 Inhibitors May Predispose to Ketoacidosis
- PMID: 26086329
- PMCID: PMC4525004
- DOI: 10.1210/jc.2015-1884
SGLT2 Inhibitors May Predispose to Ketoacidosis
Abstract
Context: Sodium glucose cotransporter 2 (SGLT2) inhibitors are antidiabetic drugs that increase urinary excretion of glucose, thereby improving glycemic control and promoting weight loss. Since approval of the first-in-class drug in 2013, data have emerged suggesting that these drugs increase the risk of diabetic ketoacidosis. In May 2015, the Food and Drug Administration issued a warning that SGLT2 inhibitors may lead to ketoacidosis.
Evidence acquisition: Using PubMed and Google, we conducted Boolean searches including terms related to ketone bodies or ketoacidosis with terms for SGLT2 inhibitors or phlorizin. Priority was assigned to publications that shed light on molecular mechanisms whereby SGLT2 inhibitors could affect ketone body metabolism.
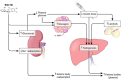
Evidence synthesis: SGLT2 inhibitors trigger multiple mechanisms that could predispose to diabetic ketoacidosis. When SGLT2 inhibitors are combined with insulin, it is often necessary to decrease the insulin dose to avoid hypoglycemia. The lower dose of insulin may be insufficient to suppress lipolysis and ketogenesis. Furthermore, SGLT2 is expressed in pancreatic α-cells, and SGLT2 inhibitors promote glucagon secretion. Finally, phlorizin, a nonselective inhibitor of SGLT family transporters decreases urinary excretion of ketone bodies. A decrease in the renal clearance of ketone bodies could also increase the plasma ketone body levels.
Conclusions: Based on the physiology of SGLT2 and the pharmacology of SGLT2 inhibitors, there are several biologically plausible mechanisms whereby this class of drugs has the potential to increase the risk of developing diabetic ketoacidosis. Future research should be directed toward identifying which patients are at greatest risk for this side effect and also to optimizing pharmacotherapy to minimize the risk to patients.
Figures
Comment in
-
Letter to the Editor: Comment on "SGLT2 inhibitors may predispose to ketoacidosis" by Taylor S.I., et al.J Clin Endocrinol Metab. 2016 Feb;101(2):L27-8. doi: 10.1210/jc.2015-4236. J Clin Endocrinol Metab. 2016. PMID: 26840113 No abstract available.
-
Response to the Letter: Comment to letter by Segar L.J Clin Endocrinol Metab. 2016 Feb;101(2):L29. doi: 10.1210/jc.2016-1021. J Clin Endocrinol Metab. 2016. PMID: 26840114 Free PMC article. No abstract available.
References
-
- Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. - PubMed
-
- Food and Drug Administration. FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm Accessed May 25, 2015.
-
- Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–1483. - PubMed
-
- St Hilaire R, Costello H. Prescriber beware: report of adverse effect of sodium-glucose cotransporter 2 inhibitor use in a patient with a contraindication. Am J Emerg Med. 2014;33:604.e3–604.e4. - PubMed
-
- JDRF. http://typeonenation.org/groups/adults/forum/topic/invokana-for-t1d/ Accessed March 3, 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

