Maleic Acid--but Not Structurally Related Methylmalonic Acid--Interrupts Energy Metabolism by Impaired Calcium Homeostasis
- PMID: 26086473
- PMCID: PMC4473014
- DOI: 10.1371/journal.pone.0128770
Maleic Acid--but Not Structurally Related Methylmalonic Acid--Interrupts Energy Metabolism by Impaired Calcium Homeostasis
Abstract
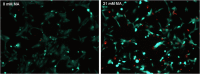
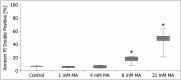
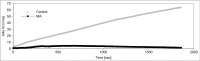
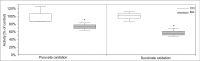
Maleic acid (MA) has been shown to induce Fanconi syndrome via disturbance of renal energy homeostasis, though the underlying pathomechanism is still under debate. Our study aimed to examine the pathomechanism underlying maleic acid-induced nephrotoxicity. Methylmalonic acid (MMA) is structurally similar to MA and accumulates in patients affected with methymalonic aciduria, a defect in the degradation of branched-chain amino acids, odd-chain fatty acids and cholesterol, which is associated with the development of tubulointerstitial nephritis resulting in chronic renal failure. We therefore used MMA application as a control experiment in our study and stressed hPTECs with MA and MMA to further validate the specificity of our findings. MMA did not show any toxic effects on proximal tubule cells, whereas maleic acid induced concentration-dependent and time-dependent cell death shown by increased lactate dehydrogenase release as well as ethidium homodimer and calcein acetoxymethyl ester staining. The toxic effect of MA was blocked by administration of single amino acids, in particular L-alanine and L-glutamate. MA application further resulted in severe impairment of cellular energy homeostasis on the level of glycolysis, respiratory chain, and citric acid cycle resulting in ATP depletion. As underlying mechanism we could identify disturbance of calcium homeostasis. MA toxicity was critically dependent on calcium levels in culture medium and blocked by the extra- and intracellular calcium chelators EGTA and BAPTA-AM respectively. Moreover, MA-induced cell death was associated with activation of calcium-dependent calpain proteases. In summary, our study shows a comprehensive pathomechanistic concept for MA-induced dysfunction and damage of human proximal tubule cells.
Conflict of interest statement
Figures














References
-
- Niaudet P, Rotig A. The kidney in mitochondrial cytopathies. Kidney international. 1997;51(4):1000–7. Epub 1997/04/01. PubMed PMID: . - PubMed
-
- Rotig A, Goutieres F, Niaudet P, Rustin P, Chretien D, Guest G, et al. Deletion of mitochondrial DNA in patient with chronic tubulointerstitial nephritis. The Journal of pediatrics. 1995;126(4):597–601. Epub 1995/04/01. PubMed PMID: . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

