State Legislation, Regulations, and Hospital Guidelines for Newborn Screening for Critical Congenital Heart Defects - United States, 2011-2014
- PMID: 26086632
- PMCID: PMC4584733
State Legislation, Regulations, and Hospital Guidelines for Newborn Screening for Critical Congenital Heart Defects - United States, 2011-2014
Abstract
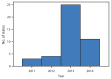
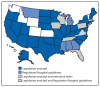
Critical congenital heart defects (CCHD) occur in approximately two of every 1,000 live births. Newborn screening provides an opportunity for reducing infant morbidity and mortality. In September 2011, the U.S. Department of Health and Human Services (HHS) Secretary endorsed the recommendation that critical congenital heart defects be added to the Recommended Uniform Screening Panel (RUSP) for all newborns. In 2014, CDC collaborated with the American Academy of Pediatrics (AAP) Division of State Government Affairs and the Newborn Screening Technical Assistance and Evaluation Program (NewSTEPs) to assess states' actions for adopting newborn screening for CCHD. Forty-three states have taken action toward newborn screening for CCHD through legislation, regulations, or hospital guidelines. Among those 43, 32 (74%) are collecting or planning to collect CCHD screening data; however, the type of data collected by CCHD newborn screening programs varies by state. State mandates for newborn screening for CCHD will likely increase the number of newborns screened, allowing for the possibility of early identification and prevention of morbidity and mortality. Data collection at the state level is important for surveillance, monitoring of outcomes, and evaluation of state CCHD newborn screening programs.
Figures
References
-
- Mahle WT, Newburger JW, Matherne GP, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology And Cardiac Surgery; Committee On Fetus And Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124:823–36. - PubMed
-
- Pass KA, Lane PA, Fernhoff PM, et al. Statement of the Council of Regional Networks for Genetic Services (CORN). US newborn screening system guidelines II: follow-up of children, diagnosis, management, and evaluation. J Pediatr. 2000;137(4 Suppl):S1–46. - PubMed
-
- Calonge N, Green NS, Rinaldo P, et al. Advisory Committee on Heritable Disorders in Newborns and Children. Committee report: method for evaluating conditions nominated for population-based screening of newborns and children. Genet Med. 2010;12:153–9. - PubMed
-
- Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. HHS Secretary adopts recommendation to add critical congenital heart disease to the Recommended Uniform Screening Panel. Washington, DC: US Department of Health and Human Services; Sep 21, 2011. 2011. Available at http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/r....
-
- Therrell BL., Jr U.S. newborn screening policy dilemmas for the twenty-first century. Mol Genet Metab. 2001;74:64–74. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

