Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers
- PMID: 26086854
- PMCID: PMC4472786
- DOI: 10.1371/journal.pone.0130142
Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers
Abstract
Background: The potential predictive role of programmed death-ligand-1 (PD-L1) expression on tumor cells in the context of solid tumor treated with checkpoint inhibitors targeting the PD-1 pathway represents an issue for clinical research.
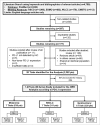
Methods: Overall response rate (ORR) was extracted from phase I-III trials investigating nivolumab, pembrolizumab and MPDL3280A for advanced melanoma, non-small cell lung cancer (NSCLC) and genitourinary cancer, and cumulated by adopting a fixed and random-effect model with 95% confidence interval (CI). Interaction test according to tumor PD-L1 was accomplished. A sensitivity analysis according to adopted drug, tumor type, PD-L1 cut-off and treatment line was performed.
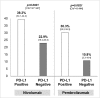
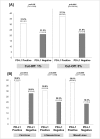
Results: Twenty trials (1,475 patients) were identified. A significant interaction (p<0.0001) according to tumor PD-L1 expression was found in the overall sample with an ORR of 34.1% (95% CI 27.6-41.3%) in the PD-L1 positive and 19.9% (95% CI 15.4-25.3%) in the PD-L1 negative population. ORR was significantly higher in PD-L1 positive in comparison to PD-L1 negative patients for nivolumab and pembrolizumab, with an absolute difference of 16.4% and 19.5%, respectively. A significant difference in activity of 22.8% and 8.7% according to PD-L1 was found for melanoma and NSCLC, respectively, with no significant difference for genitourinary cancer.
Conclusion: Overall, the three antibodies provide a significant differential effect in terms of activity according to PD-L1 expression on tumor cells. The predictive value of PD-L1 on tumor cells seems to be more robust for anti-PD-1 antibody (nivolumab and pembrolizumab), and in the context of advanced melanoma and NSCLC.
Conflict of interest statement
Figures


References
-
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. - PubMed
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

