Cysteine-independent Catalase-like Activity of Vertebrate Peroxiredoxin 1 (Prx1)
- PMID: 26088136
- PMCID: PMC4528153
- DOI: 10.1074/jbc.M115.659011
Cysteine-independent Catalase-like Activity of Vertebrate Peroxiredoxin 1 (Prx1)
Retraction in
-
Cysteine-independent catalase-like activity of vertebrate peroxiredoxin 1 (Prx1).J Biol Chem. 2015 Nov 6;290(45):27013. doi: 10.1074/jbc.A115.659011. J Biol Chem. 2015. PMID: 26546692 Free PMC article. No abstract available.
Abstract
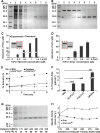
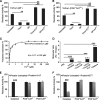
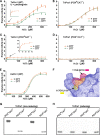
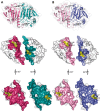
Peroxiredoxins (Prxs) are a ubiquitous family of antioxidant proteins that are known as thioredoxin peroxidases. Here we report that Prx1 proteins from Tetraodon nigroviridis and humans also possess a previously unknown catalase-like activity that is independent of Cys residues and reductants but dependent on iron. We identified that the GVL motif was essential to the catalase (CAT)-like activity of Prx1 but not to the Cys-dependent thioredoxin peroxidase (POX) activity, and we generated mutants lacking POX and/or CAT activities for individually delineating their functional features. We discovered that the TnPrx1 POX and CAT activities possessed different kinetic features in reducing H2O2. The overexpression of wild-type TnPrx1 and mutants differentially regulated the intracellular levels of reactive oxygen species and p38 phosphorylation in HEK-293T cells treated with H2O2. These observations suggest that the dual antioxidant activities of Prx1 may be crucial for organisms to mediate intracellular redox homeostasis.
Keywords: catalase; hydrogen peroxide; peroxidase; peroxiredoxin; signaling.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- McGonigle S., Dalton J. P., James E. R. (1998) Peroxidoxins: a new antioxidant family. Parasitol. Today 14, 139–145 - PubMed
-
- Kang S. W., Chae H. Z., Seo M. S., Kim K., Baines I. C., Rhee S. G. (1998) Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J. Biol. Chem. 273, 6297–6302 - PubMed
-
- Mitsumoto A., Takanezawa Y., Okawa K., Iwamatsu A., Nakagawa Y. (2001) Variants of peroxiredoxins expression in response to hydroperoxide stress. Free Radic. Biol. Med. 30, 625–635 - PubMed
-
- Rhee S. G., Kang S. W., Jeong W., Chang T. S., Yang K. S., Woo H. A. (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 - PubMed
Publication types
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

