Hydromethylation of Unactivated Olefins
- PMID: 26088401
- PMCID: PMC4490774
- DOI: 10.1021/jacs.5b05144
Hydromethylation of Unactivated Olefins
Abstract
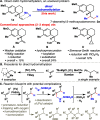
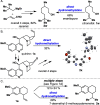
A solution to the classic unsolved problem of olefin hydromethylation is presented. This highly chemoselective method can tolerate labile and reactive chemical functionalities and uses a simple set of reagents. An array of olefins, including mono-, di-, and trisubstituted olefins, are all smoothly hydromethylated. This mild protocol can be used to simplify the synthesis of a specific target or to directly "edit" complex natural products and other advanced materials. The method is also amenable to the simple installation of radioactive and stable labeled methyl groups.
Figures


References
-
- Fontaine F.-G.; Tilley T. D. Organometallics 2005, 24, 4340.
- Terao J.; Watanabe T.; Saito K.; Kambe N.; Sonoda N. Tetrahedron Lett. 1998, 39, 9201.
- Parnes Z. N.; Bolestova G. I.; Akhrem I. S. J. Chem. Soc., Chem. Commun. 1980, 748.
-
- Isayama S.; Mukaiyama T. Chem. Lett. 1989, 1071.
- Mukaiyama T.; Isayama S.; Inoki S.; Kato K.; Yamada T.; Takai T. Chem. Lett. 1989, 449.
- Inoki S.; Kato K.; Takai T.; Isayama S.; Yamada T.; Mukaiyama T. Chem. Lett. 1989, 515.
- Kato K.; Mukaiyama T. Chem. Lett. 1992, 1137.
-
- Zombeck A.; Hamilton D. E.; Drago R. S. J. Am. Chem. Soc. 1982, 104, 6782.
- Waser J.; Carreira E. M. J. Am. Chem. Soc. 2004, 126, 5676. - PubMed
- Waser J.; Nambu H.; Carreira E. M. J. Am. Chem. Soc. 2005, 127, 8294. - PubMed
- Waser J.; González-Gómez J. C.; Nambu H.; Huber P.; Carreira E. M. Org. Lett. 2005, 7, 4249. - PubMed
- Waser J.; Gaspar B.; Nambu H.; Carreira E. M. J. Am. Chem. Soc. 2006, 128, 11693. - PubMed
- Gaspar B.; Carreira E. M. Angew. Chem., Int. Ed. 2007, 46, 4519. - PubMed
- Gaspar B.; Carreira E. M. Angew. Chem., Int. Ed. 2008, 47, 5758. - PubMed
- Gaspar B.; Carreira E. M. J. Am. Chem. Soc. 2009, 131, 13214. - PubMed
- Shigehisa H.; Koseki N.; Shimizu N.; Fujisawa M.; Niitsu M.; Hiroya K. J. Am. Chem. Soc. 2014, 136, 13534. - PubMed
-
- Magnus P.; Payne A. H.; Waring M. J.; Scott D. A.; Lynch V. Tetrahedron Lett. 2000, 41, 9725.
- Waser J.; Carreira E. M. Angew. Chem., Int. Ed. 2004, 43, 4099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

