In vivo detection of clinically non-apparent ocular surface inflammation in patients with meibomian gland dysfunction-associated refractory dry eye symptoms: a pilot study
- PMID: 26088680
- PMCID: PMC4541343
- DOI: 10.1038/eye.2015.103
In vivo detection of clinically non-apparent ocular surface inflammation in patients with meibomian gland dysfunction-associated refractory dry eye symptoms: a pilot study
Abstract
Purpose: The utility of in vivo confocal microscopy (IVCM) in the investigation of palpebral conjunctival and corneal inflammation in patients with meibomian gland dysfunction (MGD)-associated refractory dry eye symptoms following gland expression, despite objective clinical improvement.
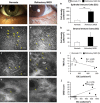
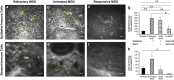
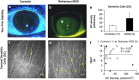
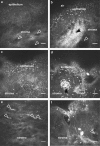
Methods: A retrospective, observational pilot study was conducted evaluating five patients with MGD-associated refractory dry eye symptoms and three control groups: symptomatic untreated MGD patients (n=3), treatment-responsive MGD patients with improved symptoms (n=3) and asymptomatic healthy normals (n=11). Ocular surface disease index (OSDI) scores, tear break-up time (TBUT), the number of meibomian glands yielding liquid secretion (MGYLS), palpebral conjunctival epithelial and substantia propria immune cell (EIC, SIC), and corneal dendritic cell (DC) densities were measured.
Results: Despite clinical improvement (TBUT: 6.4±1.2 s to 10.1±2.1 s, P=0.03; MGYLS: 3.5±0.8 glands to 7.0±1.1 glands, P=0.13) and a normal clinical examination post treatment, MGD patients remained symptomatic. IVCM revealed increased immune cells in the palpebral conjunctiva (refractory MGD EIC=592.6±110.1 cells/mm2 untreated MGD EIC=522.6±104.7 cells/mm2, P=0.69; responsive MGD EIC=194.9±119.4 cells/mm2, P<0.01; normals EIC=123.7±19.2 cells/mm2, P< 0.001), but not the cornea (refractory MGD DC=60.9±28.3 cells/mm2; normals DC=25.9±6.3 cells/mm2; P=0.43). EIC did not correlate with TBUT (Rs=-0.26, P=0.33). OSDI scores correlated with both EIC (Rs=0.76, P<0.001) and TBUT (Rs=-0.69, P<0.01) but not SIC. Intraglandular immune cells were also seen.
Conclusion: MGD-associated refractory symptoms and the symptom-sign disparity may be explained by clinically non-apparent, active inflammation of the palpebral conjunctiva as detected by IVCM. These patients may benefit from anti-inflammatory therapy.
Figures
References
-
- Viso E, Gude F, Rodriguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population-based study in Spain. Cornea. 2011;30 (1:1–6. - PubMed
-
- Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2 (2:149–165. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

