Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles
- PMID: 26088982
- PMCID: PMC4493852
- DOI: 10.1186/1556-276X-9-684
Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles
Abstract
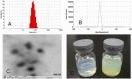
To improve its poor aqueous solubility and stability, the potential chemotherapeutic drug quercetin was encapsulated in soluplus polymeric micelles by a modified film dispersion method. With the encapsulation efficiency over 90%, the quercetin-loaded polymeric micelles (Qu-PMs) with drug loading of 6.7% had a narrow size distribution around mean size of 79.00 ± 2.24 nm, suggesting the complete dispersibility of quercetin in water. X-ray diffraction (XRD) patterns illustrated that quercetin was in amorphous or molecular form within PMs. Fourier transform infrared spectroscopy (FTIR) indicated that quercetin formed intermolecular hydrogen bonding with carriers. An in vitro dialysis test showed the Qu-PMs possessed significant sustained-release property, and the formulation was stable for at least 6 months under accelerated conditions. The pharmacokinetic study in beagle dogs showed that absorption of quercetin after oral administration of Qu-PMs was improved significantly, with a half-life 2.19-fold longer and a relative oral bioavailability of 286% as compared to free quercetin. Therefore, these novel soluplus polymeric micelles can be applied to encapsulate various poorly water-soluble drugs towards a development of more applicable therapeutic formulations.
Figures






References
-
- Lavellea EC, Sharif S, Thomas NW, Holland J, Davis SS. The importance of gastrointestinal uptake of particles in the design of oral delivery systems. Adv Drug Delivery Rev. 1995;18:5–22. doi: 10.1016/0169-409X(95)00048-C. - DOI
-
- Deshpande AA, Rhodes CT, Shah NH, Malick AW. Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm. 1996;22:531–539. doi: 10.3109/03639049609108355. - DOI
-
- Johnston APR, Such GK, Ng SL, Caruso F. Challenges facing colloidal delivery systems: FROM synthesis to the clinic. Curr Opin Colloid Interface Sci. 2011;16:171–181. doi: 10.1016/j.cocis.2010.11.003. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

