Analysis of type II diabetes mellitus adipose-derived stem cells for tissue engineering applications
- PMID: 26090087
- PMCID: PMC4456321
- DOI: 10.1177/2041731415579215
Analysis of type II diabetes mellitus adipose-derived stem cells for tissue engineering applications
Abstract
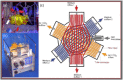
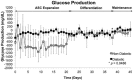
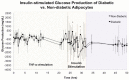
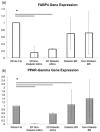
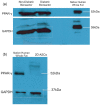
To address the functionality of diabetic adipose-derived stem cells in tissue engineering applications, adipose-derived stem cells isolated from patients with and without type II diabetes mellitus were cultured in bioreactor culture systems. The adipose-derived stem cells were differentiated into adipocytes and maintained as functional adipocytes. The bioreactor system utilizes a hollow fiber-based technology for three-dimensional perfusion of tissues in vitro, creating a model in which long-term culture of adipocytes is feasible, and providing a potential tool useful for drug discovery. Daily metabolic activity of the adipose-derived stem cells was analyzed within the medium recirculating throughout the bioreactor system. At experiment termination, tissues were extracted from bioreactors for immunohistological analyses in addition to gene and protein expression. Type II diabetic adipose-derived stem cells did not exhibit significantly different glucose consumption compared to adipose-derived stem cells from patients without type II diabetes (p > 0.05, N = 3). Expression of mature adipocyte genes was not significantly different between diabetic/non-diabetic groups (p > 0.05, N = 3). Protein expression of adipose tissue grown within all bioreactors was verified by Western blotting.The results from this small-scale study reveal adipose-derived stem cells from patients with type II diabetes when removed from diabetic environments behave metabolically similar to the same cells of non-diabetic patients when cultured in a three-dimensional perfusion bioreactor, suggesting that glucose transport across the adipocyte cell membrane, the hindrance of which being characteristic of type II diabetes, is dependent on environment. The presented observation describes a tissue-engineered tool for long-term cell culture and, following future adjustments to the culture environment and increased sample sizes, potentially for anti-diabetic drug testing.
Keywords: Adipocytes; adipogenesis; bioreactor; cell and tissue culture; cell cultures; diabetes research; mesenchymal stem cells; stem cells—adipose; three-dimensional; tissue culture models; tissue engineering.
Conflict of interest statement
Figures



References
-
- Center for Disease Control. Adult obesity facts, 13 August 2012, http://www.cdc.gov/obesity/data/adult.html (accessed 6 November 2012).
-
- Center for Disease Control. National diabetes fact sheet, 2011, http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (accessed 6 November 2012).
-
- Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54(2): S97–S107. - PubMed
-
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83: 461S–4615S. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

