Non-VKA Oral Anticoagulants: Accurate Measurement of Plasma Drug Concentrations
- PMID: 26090400
- PMCID: PMC4452246
- DOI: 10.1155/2015/345138
Non-VKA Oral Anticoagulants: Accurate Measurement of Plasma Drug Concentrations
Abstract
Non-VKA oral anticoagulants (NOACs) have now widely reached the lucrative market of anticoagulation. While the marketing authorization holders claimed that no routine monitoring is required and that these compounds can be given at fixed doses, several evidences arisen from the literature tend to demonstrate the opposite. New data suggests that an assessment of the response at the individual level could improve the benefit-risk ratio of at least dabigatran. Information regarding the association of rivaroxaban and apixaban exposure and the bleeding risk is available in the drug approval package on the FDA website. These reviews suggest that accumulation of these compounds increases the risk of experiencing a bleeding complication. Therefore, in certain patient populations such as patients with acute or chronic renal impairment or with multiple drug interactions, measurement of drug exposure may be useful to ensure an optimal treatment response. More specific circumstances such as patients experiencing a haemorrhagic or thromboembolic event during the treatment duration, patients who require urgent surgery or an invasive procedure, or patient with a suspected overdose could benefit from such a measurement. This paper aims at providing guidance on how to best estimate the intensity of anticoagulation using laboratory assays in daily practice.
Figures
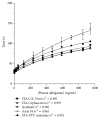
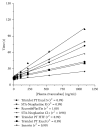
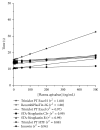
References
-
- Bloemen S., De Laat M., De Laat B., Hemker H. C., Al Dieri R. Will one size of anticoagulant dosage fit all? Drug Development Research. 2013;74(7):406–412. doi: 10.1002/ddr.21097. - DOI
-
- European Medicine Agency-Xarelto. Summary of Product Characteristics. 2013, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- European Medicine Agency-Pradaxa. Summary of Product Characteristics. 2014, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- European Medicine Agency. Eliquis: Summary of Product Characteristics 2014, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

