Characterization of a Thioredoxin-1 Gene from Taenia solium and Its Encoding Product
- PMID: 26090410
- PMCID: PMC4452251
- DOI: 10.1155/2015/453469
Characterization of a Thioredoxin-1 Gene from Taenia solium and Its Encoding Product
Abstract
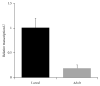
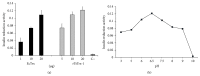
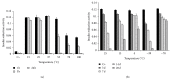
Taenia solium thioredoxin-1 gene (TsTrx-1) has a length of 771 bp with three exons and two introns. The core promoter gene presents two putative stress transcription factor binding sites, one putative TATA box, and a transcription start site (TSS). TsTrx-1 mRNA is expressed higher in larvae than in adult. This gene encodes a protein of 107 amino acids that presents the Trx active site (CGPC), the classical secondary structure of the thioredoxin fold, and the highest degree of identity with the Echinococcus granulosus Trx. A recombinant TsTrx-1 (rTsTrx-1) was produced in Escherichia coli with redox activity. Optimal activity for rTsTrx-1 was at pH 6.5 in the range of 15 to 25°C. The enzyme conserved activity for 3 h and lost it in 24 h at 37°C. rTsTrx-1 lost 50% activity after 1 h and lost activity completely in 24 h at temperatures higher than 55°C. Best storage temperature for rTsTrx-1 was at -70°C. It was inhibited by high concentrations of H₂O₂ and methylglyoxal (MG), but it was inhibited neither by NaCl nor by anti-rTsTrx-1 rabbit antibodies that strongly recognized a ~12 kDa band in extracts from several parasites. These TsTrx-1 properties open the opportunity to study its role in relationship T. solium-hosts.
Figures




References
-
- Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7017–7021. doi: 10.1073/pnas.91.15.7017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

