Trypanosoma cruzi Infection through the Oral Route Promotes a Severe Infection in Mice: New Disease Form from an Old Infection?
- PMID: 26090667
- PMCID: PMC4474863
- DOI: 10.1371/journal.pntd.0003849
Trypanosoma cruzi Infection through the Oral Route Promotes a Severe Infection in Mice: New Disease Form from an Old Infection?
Abstract
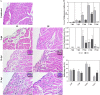
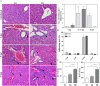
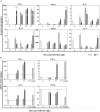
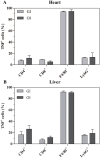
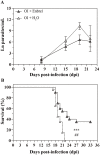
Oral transmission of Chagas disease has been documented in Latin American countries. Nevertheless, significant studies on the pathophysiology of this form of infection are largely lacking. The few studies investigating oral route infection disregard that inoculation in the oral cavity (Oral infection, OI) or by gavage (Gastrointestinal infection, GI) represent different infection routes, yet both show clear-cut parasitemia and heart parasitism during the acute infection. Herein, BALB/c mice were subjected to acute OI or GI infection using 5x10(4) culture-derived Trypanosoma cruzi trypomastigotes. OI mice displayed higher parasitemia and mortality rates than their GI counterparts. Heart histopathology showed larger areas of infiltration in the GI mice, whereas liver lesions were more severe in the OI animals, accompanied by higher Alanine Transaminase and Aspartate Transaminase serum contents. A differential cytokine pattern was also observed because OI mice presented higher pro-inflammatory cytokine (IFN-γ, TNF) serum levels than GI animals. Real-time PCR confirmed a higher TNF, IFN-γ, as well as IL-10 expression in the cardiac tissue from the OI group compared with GI. Conversely, TGF-β and IL-17 serum levels were greater in the GI animals. Immunolabeling revealed macrophages as the main tissue source of TNF in infected mice. The high mortality rate observed in the OI mice paralleled the TNF serum rise, with its inhibition by an anti-TNF treatment. Moreover, differences in susceptibility between GI versus OI mice were more clearly related to the host response than to the effect of gastric pH on parasites, since infection in magnesium hydroxide-treated mice showed similar results. Overall, the present study provides conclusive evidence that the initial site of parasite entrance critically affects host immune response and disease outcome. In light of the occurrence of oral Chagas disease outbreaks, our results raise important implications in terms of the current view of the natural disease course and host-parasite relationship.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Oral Versus Intragastric Inoculation: Similar Pathways of Trypanosoma cruzi Experimental Infection? From Target Tissues, Parasite Evasion, and Immune Response.Front Immunol. 2018 Jul 27;9:1734. doi: 10.3389/fimmu.2018.01734. eCollection 2018. Front Immunol. 2018. PMID: 30100907 Free PMC article. Review.
-
Dogs infected with the blood trypomastigote form of Trypanosoma cruzi display an increase expression of cytokines and chemokines plus an intense cardiac parasitism during acute infection.Mol Immunol. 2014 Mar;58(1):92-7. doi: 10.1016/j.molimm.2013.11.007. Epub 2013 Dec 7. Mol Immunol. 2014. PMID: 24317279
-
Coinfection with different Trypanosoma cruzi strains interferes with the host immune response to infection.PLoS Negl Trop Dis. 2010 Oct 12;4(10):e846. doi: 10.1371/journal.pntd.0000846. PLoS Negl Trop Dis. 2010. PMID: 20967289 Free PMC article.
-
Evolution of infection in mice inoculated by the oral route with different developmental forms of Trypanosoma cruzi I and II.Exp Parasitol. 2013 Nov;135(3):511-7. doi: 10.1016/j.exppara.2013.08.013. Epub 2013 Aug 29. Exp Parasitol. 2013. PMID: 23994765
-
[Role of cytokines in resistance and pathology in Trypanosoma cruzi infection].Rev Argent Microbiol. 1996 Apr-Jun;28(2):99-109. Rev Argent Microbiol. 1996. PMID: 8768488 Review. Spanish.
Cited by
-
Central nervous system commitment in Chagas disease.Front Immunol. 2022 Nov 10;13:975106. doi: 10.3389/fimmu.2022.975106. eCollection 2022. Front Immunol. 2022. PMID: 36439149 Free PMC article. Review.
-
The importance of estimating the burden of disease from foodborne transmission of Trypanosoma cruzi.PLoS Negl Trop Dis. 2024 Feb 8;18(2):e0011898. doi: 10.1371/journal.pntd.0011898. eCollection 2024 Feb. PLoS Negl Trop Dis. 2024. PMID: 38329945 Free PMC article. Review.
-
Correlation of transforming growth factor-β1 and tumour necrosis factor levels with left ventricular function in Chagas disease.Mem Inst Oswaldo Cruz. 2018 Feb 19;113(4):e170440. doi: 10.1590/0074-02760170440. Mem Inst Oswaldo Cruz. 2018. PMID: 29513876 Free PMC article.
-
Sexual dimorphism-driven differences are overcome in a preclinical vaccine model against Trypanosoma cruzi.Front Immunol. 2025 Jun 26;16:1526573. doi: 10.3389/fimmu.2025.1526573. eCollection 2025. Front Immunol. 2025. PMID: 40642088 Free PMC article.
-
Electrophysiology Models for End-Stage Renal Disease Maladaptations That Promote Asystole.Rev Cardiovasc Med. 2025 Jul 30;26(7):37400. doi: 10.31083/RCM37400. eCollection 2025 Jul. Rev Cardiovasc Med. 2025. PMID: 40776941 Free PMC article. Review.
References
-
- da Silva NN, Clausell DT, Nolibos H, de Mello AL, Ossanai J, et al. (1968) [Epidemic outbreak of Chagas disease probably due to oral contamination]. Rev Inst Med Trop Sao Paulo 10: 265–276. - PubMed
-
- Toso MA, Vial UF, Galanti N (2011) [Oral transmission of Chagas' disease]. Rev Med Chil 139: 258–266. doi: /S0034-98872011000200017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

