Constitutive Activity in an Ancestral Form of Abl Tyrosine Kinase
- PMID: 26090675
- PMCID: PMC4474922
- DOI: 10.1371/journal.pone.0131062
Constitutive Activity in an Ancestral Form of Abl Tyrosine Kinase
Abstract
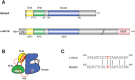
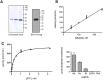
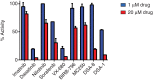
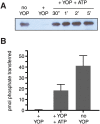
The c-abl proto-oncogene encodes a nonreceptor tyrosine kinase that is found in all metazoans, and is ubiquitously expressed in mammalian tissues. The Abl tyrosine kinase plays important roles in the regulation of mammalian cell physiology. Abl-like kinases have been identified in the genomes of unicellular choanoflagellates, the closest relatives to the Metazoa, and in related unicellular organisms. Here, we have carried out the first characterization of a premetazoan Abl kinase, MbAbl2, from the choanoflagellate Monosiga brevicollis. The enzyme possesses SH3, SH2, and kinase domains in a similar arrangement to its mammalian counterparts, and is an active tyrosine kinase. MbAbl2 lacks the N-terminal myristoylation and cap sequences that are critical regulators of mammalian Abl kinase activity, and we show that MbAbl2 is constitutively active. When expressed in mammalian cells, MbAbl2 strongly phosphorylates cellular proteins on tyrosine, and transforms cells much more potently than mammalian Abl kinase. Thus, MbAbl2 appears to lack the autoinhibitory mechanism that tightly constrains the activity of mammalian Abl kinases, suggesting that this regulatory apparatus arose more recently in metazoan evolution.
Conflict of interest statement
Figures
References
-
- Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. (2003) Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112: 859–871. - PubMed
-
- Wong S, Witte ON (2004) The BCR-ABL story: bench to bedside and back. Annu Rev Immunol 22: 247–306. - PubMed
-
- Daley GQ, Van Etten RA, Baltimore D (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247: 824–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

