Projected impact of Cervarix™ vaccination on oncogenic human papillomavirus infection and cervical cancer in the United Kingdom
- PMID: 26090944
- PMCID: PMC5155626
- DOI: 10.1080/21645515.2015.1054584
Projected impact of Cervarix™ vaccination on oncogenic human papillomavirus infection and cervical cancer in the United Kingdom
Abstract
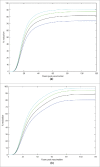
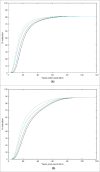
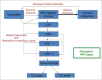
We developed a dynamic compartmental model to assess the impact of HPV Universal Mass Vaccination (UMV) with Cervarix™, which offers protection against HPV16/18 and cross-protection against other cancer-causing types, using up-to-date efficacy data. Analyses were performed in the UK because of the large amount of high quality epidemiological data available. For each HPV type/group of types considered, the model was calibrated to 14 epidemiological datasets (prevalence of HPV infection, cervical intraepithelial neoplasia (CIN): CIN1, CIN2, CIN3 pre-screening and cervical cancer (CC) incidence over 10 y post-screening). Impacts of cross-protection, female catch-up vaccination, and additional male vaccination on oncogenic infections, high-grade CIN (CIN2+) and CC were evaluated. Our results show that female UMV with 80% coverage and cross-protection against high-risk types resulted in 81% CIN2+ and 88% CC reductions vs. 57% and 75%, respectively, without cross-protection. Vaccinating 40% of males and 80% of females was equivalent to 90% female-only coverage regarding CIN2+ (87% and 87%, respectively) and CC (93% and 94%, respectively) reductions. Female-only coverage of 80% substantially reduced male HPV16 and 18 infection due to herd protection (74% and 89%, respectively). Increasing female coverage to 90% reduced HPV16 and HPV18 infections in males relatively similarly to 80% female combined with 40% male coverage. Model outcomes strengthen previous conclusions about the significant added value of Cervarix™ cross-protection for CC prevention, the primary HPV vaccination public health priority. Regarding female CC prevention and male HPV16/18 infection, small increases in female coverage induce similar benefits to those achieved by additionally vaccinating men with 40% coverage.
Keywords: Cervarix™; HPV; cervical cancer; cross-protection; universal mass vaccination.
Figures





Comment in
-
Clarification on the impact of cervarix vaccination on human papillomavirus infection and cervical cancer in the United Kingdom.Hum Vaccin Immunother. 2016 Jul 2;12(7):1940-2. doi: 10.1080/21645515.2016.1143159. Hum Vaccin Immunother. 2016. PMID: 26857560 Free PMC article. No abstract available.
-
Response letter regarding the letter to the editors by Brown et al.Hum Vaccin Immunother. 2016 Jul 2;12(7):1943-6. doi: 10.1080/21645515.2016.1151598. Epub 2016 May 10. Hum Vaccin Immunother. 2016. PMID: 27163545 Free PMC article. No abstract available.
References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12-9; PMID:10451482; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F - DOI - PubMed
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010; 46:765-81; PMID:20116997; http://dx.doi.org/ 10.1016/j.ejca.2009.12.014 - DOI - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and highgrade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121:621-32; PMID:17405118; http://dx.doi.org/ 10.1002/ijc.22527 - DOI - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al.. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 - DOI - PubMed
-
- Ault KA; Future II Study Group . Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet 2007; 369:1861-8; PMID:17544766; http://dx.doi.org/ 10.1016/S0140-6736(07)60852-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
