Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer
- PMID: 26091251
- PMCID: PMC4613597
- DOI: 10.1080/15384101.2015.1005530
Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer
Abstract
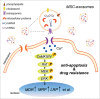
Mesenchymal stem cells (MSCs) play an important role in chemoresistance. Exosomes have been reported to modify cellular phenotype and function by mediating cell-cell communication. In this study, we aimed to investigate whether exosomes derived from MSCs (MSC-exosomes) are involved in mediating the resistance to chemotherapy in gastric cancer and to explore the underlying molecular mechanism. We found that MSC-exosomes significantly induced the resistance of gastric cancer cells to 5-fluorouracil both in vivo and ex vivo. MSC-exosomes antagonized 5-fluorouracil-induced apoptosis and enhanced the expression of multi-drug resistance associated proteins, including MDR, MRP and LRP. Mechanistically, MSC-exosomes triggered the activation of calcium/calmodulin-dependent protein kinases (CaM-Ks) and Raf/MEK/ERK kinase cascade in gastric cancer cells. Blocking the CaM-Ks/Raf/MEK/ERK pathway inhibited the promoting role of MSC-exosomes in chemoresistance. Collectively, MSC-exosomes could induce drug resistance in gastric cancer cells by activating CaM-Ks/Raf/MEK/ERK pathway. Our findings suggest that MSC-exosomes have profound effects on modifying gastric cancer cells in the development of drug resistance. Targeting the interaction between MSC-exosomes and cancer cells may help improve the efficacy of chemotherapy in gastric cancer.
Keywords: drug resistance; exosomes; gastric cancer; mesenchymal stem cells.
Figures






References
-
- Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, Gerritsen MG, Schipper HS, Backer MJ, van Amersfoort M, et al. . Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 2011; 20(3):370-83; PMID:21907927; http://dx.doi.org/10.1016/j.ccr.2011.08.010 - DOI - PubMed
-
- Cukierman E, Bassi DE. The mesenchymal tumor microenvironment: a drug-resistant niche. Cell Adh Migr 2012; 6(3):285-96; PMID:22568991; http://dx.doi.org/10.4161/cam.20210 - DOI - PMC - PubMed
-
- Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X, Xu WR. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle 2011; 10(18):3198-207; PMID:21900753; http://dx.doi.org/10.4161/cc.10.18.17638 - DOI - PubMed
-
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 2012; 315(1):28-37; PMID:22055459; http://dx.doi.org/10.1016/j.canlet.2011.10.002 - DOI - PubMed
-
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, et al. . Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013; 22(6):845-54; PMID:23002959; http://dx.doi.org/10.1089/scd.2012.0395 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
