Low expression of activation marker CD69 and chemokine receptors CCR5 and CXCR3 on memory T cells after 2009 H1N1 influenza A antigen stimulation in vitro following H1N1 vaccination of HIV-infected individuals
- PMID: 26091502
- PMCID: PMC4635846
- DOI: 10.1080/21645515.2015.1051275
Low expression of activation marker CD69 and chemokine receptors CCR5 and CXCR3 on memory T cells after 2009 H1N1 influenza A antigen stimulation in vitro following H1N1 vaccination of HIV-infected individuals
Abstract
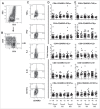
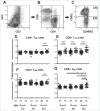
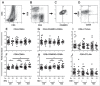
Unlike well-studied antibody responses to pandemic 2009 H1N1 influenza A virus vaccines in human immunodeficiency virus-infected (HIV+) individuals, less well understood are cell-mediated immune (CMI) responses to this antigen in this susceptible population. We investigated such influenza-specific CMI responses in 61 HIV+ individuals and in 20 HIV-negative (HIV-) healthy controls. Each was vaccinated with a single licensed dose of inactivated, split-virion vaccine comprised of the influenza A/California/7/2009 (H1N1) virus-like strain. Cells collected just prior to vaccination and at 1 and 3 months afterwards were stimulated in vitro with dialyzed vaccine antigen and assayed by flow cytometry for cytokines TNF-α, IFN-γ, IL-2, and IL-10, for degranulation marker CD107a, as well as phenotypes of memory T-cell subpopulations. Comparable increases of cytokine-producing and CD107a-expressing T cells were observed in both HIV+ subjects and healthy HIV-controls. However, by 3 months post-vaccination, in vitro antigen stimulation of peripheral blood mononuclear cells induced greater expansion in controls of both CD4 and CD8 central memory and effector memory T cells, as well as higher expression of the activation marker CD69 and chemokine receptors CCR5 and CXCR3 than in HIV+ subjects. We concluded CD4+ and CD8+ memory T cells produce cytokines at comparable levels in both groups, whereas the expression after in vitro stimulation of molecules critical for cell migration to infection sites are lower in the HIV+ than in comparable controls. Further immunization strategies against influenza are needed to improve the CMI responses in people living with HIV.
Keywords: 2009 H1N1 influenza A vaccine; HIV-infection; activation markers; cellular immunity; chemokine receptors; immunological memory responses.
Figures


References
-
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674-9; PMID:19564633; http://dx.doi.org/10.1056/NEJMoa0904023 - DOI - PubMed
-
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al.. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708-19; PMID:20445182; http://dx.doi.org/10.1056/NEJMra1000449 - DOI - PubMed
-
- Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol 1983; 37:529-49; PMID:6357060; http://dx.doi.org/10.1146/annurev.mi.37.100183.002525 - DOI - PubMed
-
- Cheong HJ, Song JY, Heo JY, Noh JY, Choi WS, Park DW, Wie SH, Kim WJ. Immunogenicity and safety of influenza A (H1N1) 2009 monovalent inactivated split vaccine in Korea. Vaccine 2010; 29:523-7; PMID:21055502; http://dx.doi.org/10.1016/j.vaccine.2010.10.060 - DOI - PubMed
-
- Lu CY, Shao PL, Chang LY, Huang YC, Chiu CH, Hsieh YC, Lin TY, Huang LM. Immunogenicity and safety of a monovalent vaccine for the 2009 pandemic influenza virus A (H1N1) in children and adolescents. Vaccine 2010; 28:5864-70; PMID:20600484; http://dx.doi.org/10.1016/j.vaccine.2010.06.059 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
