Recombinant human C1 esterase inhibitor in the management of hereditary angioedema
- PMID: 26091744
- PMCID: PMC4488495
- DOI: 10.1007/s40261-015-0300-z
Recombinant human C1 esterase inhibitor in the management of hereditary angioedema
Abstract
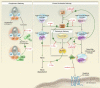
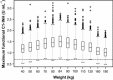
Hereditary angioedema (HAE), a rare autosomal dominant genetic disorder, is caused by a deficiency in functional C1 esterase inhibitor (C1-INH). This potentially life-threatening condition manifests as recurrent attacks of subcutaneous and submucosal swelling of the skin, gastrointestinal tract and larynx. The management of HAE includes treatment of acute episodes, short-term prophylaxis in preparation for exposure to known triggers and long-term prophylaxis to decrease the incidence and severity of HAE attacks. Four products are approved in the USA for the treatment of acute attacks of HAE, including one human plasma-derived C1-INH therapy, a recombinant human C1-INH product (rhC1-INH), a plasma kallikrein inhibitor and a bradykinin B2 receptor antagonist. In addition, one human plasma-derived C1-INH therapy and danazol are approved for prophylaxis of HAE attacks. rhC1-INH, extracted from the milk of transgenic rabbits, is a glycoprotein of 478 amino acids with an identical amino acid sequence to the endogenous human C1-INH protein. Population pharmacokinetic analysis of rhC1-INH supports an intravenous dosing strategy of 50 U/kg (maximum 4200 U). The safety and efficacy of rhC1-INH in the treatment of acute attacks in patients with HAE were demonstrated in three randomized, double-blind, placebo-controlled studies and two open-label extension studies. In a pilot prophylaxis study, weekly administration of rhC1-INH 50 U/kg for 8 weeks reduced the incidence of HAE attacks and was well tolerated. Administration of rhC1-INH has not been associated with the development of anti-drug antibodies or antibodies to anti-host-related impurities.
Figures

References
-
- Cardarelli W. Managed care implications of hereditary angioedema. Am J Manag Care. 2013;19(7 Suppl):s119–s124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

