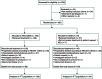
A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer
- PMID: 26091808
- PMCID: PMC4551155
- DOI: 10.1093/annonc/mdv264
A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer
Erratum in
-
A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer.Ann Oncol. 2015 Dec;26(12):2505. doi: 10.1093/annonc/mdv477. Epub 2015 Oct 21. Ann Oncol. 2015. PMID: 26489442 Free PMC article. No abstract available.
-
A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer.Ann Oncol. 2016 Jun;27(6):1180. doi: 10.1093/annonc/mdw095. Epub 2016 Mar 3. Ann Oncol. 2016. PMID: 26945010 Free PMC article. No abstract available.
Abstract
Background: Rigosertib (ON 01910.Na), a first-in-class Ras mimetic and small-molecule inhibitor of multiple signaling pathways including polo-like kinase 1 (PLK1) and phosphoinositide 3-kinase (PI3K), has shown efficacy in preclinical pancreatic cancer models. In this study, rigosertib was assessed in combination with gemcitabine in patients with treatment-naïve metastatic pancreatic adenocarcinoma.
Materials and methods: Patients with metastatic pancreatic adenocarcinoma were randomized in a 2:1 fashion to gemcitabine 1000 mg/m(2) weekly for 3 weeks of a 4-week cycle plus rigosertib 1800 mg/m(2) via 2-h continuous IV infusions given twice weekly for 3 weeks of a 4-week cycle (RIG + GEM) versus gemcitabine 1000 mg/m(2) weekly for 3 weeks in a 4-week cycle (GEM).
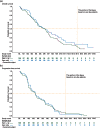
Results: A total of 160 patients were enrolled globally and randomly assigned to RIG + GEM (106 patients) or GEM (54). The most common grade 3 or higher adverse events were neutropenia (8% in the RIG + GEM group versus 6% in the GEM group), hyponatremia (17% versus 4%), and anemia (8% versus 4%). The median overall survival was 6.1 months for RIG + GEM versus 6.4 months for GEM [hazard ratio (HR), 1.24; 95% confidence interval (CI) 0.85-1.81]. The median progression-free survival was 3.4 months for both groups (HR = 0.96; 95% CI 0.68-1.36). The partial response rate was 19% versus 13% for RIG + GEM versus GEM, respectively. Of 64 tumor samples sent for molecular analysis, 47 were adequate for multiplex genetic testing and 41 were positive for mutations. The majority of cases had KRAS gene mutations (40 cases). Other mutations detected included TP53 (13 cases) and PIK3CA (1 case). No correlation between mutational status and efficacy was detected.
Conclusions: The combination of RIG + GEM failed to demonstrate an improvement in survival or response compared with GEM in patients with metastatic pancreatic adenocarcinoma. Rigosertib showed a similar safety profile to that seen in previous trials using the IV formulation.
Keywords: PI3K inhibitor; PLK1 inhibitor; Ras mimetic; pancreatic cancer; phase II/III; rigosertib.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Society AC. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
-
- Li D, Xie K, Wolff R et al. . Pancreatic cancer. Lancet 2004; 363(9414): 1049–1057. - PubMed
-
- Burris HA, Moore MJ, Andersen J et al. . Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15(6): 2403–2413. - PubMed
-
- Moore MJ, Goldstein D, Hamm J et al. . Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25(15): 1960–1966. - PubMed
-
- Conroy T, Desseigne F, Ychou M et al. . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364(19): 1817–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous