Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women
- PMID: 26091836
- PMCID: PMC10979523
- DOI: 10.1002/14651858.CD011158.pub2
Multiple micronutrient powders for home (point-of-use) fortification of foods in pregnant women
Abstract
Background: It is estimated that 32 million pregnant women suffer from anaemia worldwide. Due to increased metabolic demands, pregnant women are particularly vulnerable to anaemia and vitamin and mineral deficiencies, leading to adverse health effects in both the mother and her baby. Despite the demonstrated benefits of prenatal supplementation with iron and folic acid or multiple micronutrients, poor adherence to routine supplementation has limited the effectiveness of this intervention in many settings. Micronutrient powders for point-of-use fortification are packed, single-dose sachets containing vitamins and minerals that can be added onto prepared food to improve its nutrient profile. The use of multiple micronutrient powders for point-of-use fortification of foods in pregnant women could be an alternative intervention to prenatal micronutrient supplementation.
Objectives: To assess the effects of prenatal home (point-of-use) fortification of foods with multiple micronutrient powders on maternal and newborn health.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2015) and the International Clinical Trials Registry Platform (ICTRP) (31 January 2015). We also contacted relevant agencies to identify ongoing and unpublished studies.
Selection criteria: Randomised controlled trials (both individual and cluster randomisation) and quasi-randomised trials, irrespective of language or publication status.The intervention was micronutrient powders for point-of-use fortification of foods, containing at least three micronutrients with one of them being iron, provided to pregnant women of any gestational age and parity. Five comparison groups were considered: no intervention/placebo, iron and folic acid supplements, iron-only supplements, folic-acid only supplements, and multiple micronutrients in supplements.
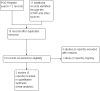
Data collection and analysis: Two review authors independently assessed the eligibility of studies, extracted and checked data accuracy, and assessed the risk of bias of included studies.
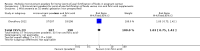
Main results: Our search identified 12 reports (relating to six studies). We included two cluster-randomised controlled trials (involving 1172 women) - these trials were considered to be at a moderate to high risk of bias due to methodological limitations. One trial is ongoing, and three studies were excluded. Micronutrient powders for point-of-use fortification of foods versus iron and folic acid supplementsOne trial (involving 478 pregnant women attending 42 antenatal care centres) compared micronutrient powders containing iron, folic acid, vitamin C and zinc with iron and folic acid tablets provided daily from 14 to 22 weeks to 32 weeks' gestation. The trial did not report on any of this review's primary outcomes: maternal anaemia at or near term, maternal iron deficiency, maternal mortality, adverse effects, low birthweight, preterm births. Nor did the trial report on the majority of this review's secondary outcomes, with the exception of maternal adherence. Adherence to micronutrient powders was lower than adherence to iron and folic acid supplements (risk ratio (RR) 0.76, 95% confidence interval (CI) 0.66 to 0.87, one study, n = 405). Micronutrient powders for point-of-use fortification of foods versus same multiple micronutrients in supplementsOne study (involving 694 pregnant women from 18 communities), compared micronutrient powders containing iron, folic acid, vitamin C, zinc, iodine, vitamin E and vitamin B12 with tablets containing the same seven micronutrients. There was no difference in maternal anaemia at 37 weeks of gestation (RR 0.92, 95% CI 0.53 to 1.59, one study, n = 470, very low quality evidence). The trial did not report on any of this review's other primary outcomes in relation to maternal iron deficiency, maternal mortality, adverse effects, low birthweight, or preterm birth. In terms of this review's secondary outcomes, the included trial did not report on the majority of this review's prespecified secondary outcomes with one exception - there was no clear difference in maternal haemoglobin Hb or near term (mean difference (MD) 1.0 g/L, 95% CI -1.77 to 3.77, one study, n = 470).
Authors' conclusions: Limited evidence suggests that micronutrient powders for point-of-use fortification of foods have no clear difference as multiple micronutrient supplements on maternal anaemia (very low quality evidence) and Hb at or near term. There is limited evidence to suggest that women were more likely to adhere to taking tablets than using micronutrient powders.The overall quality of evidence was judged very low (due to methodological limitations), and no evidence was available for the majority of primary and secondary outcomes. Therefore, more evidence is needed to assess the potential benefits or harms of the use of micronutrient powders in pregnant women on maternal and infant health outcomes. Future trials should also assess adherence to micronutrient powders and be adequately powered to evaluate the effects on birth outcomes and morbidity.
Conflict of interest statement
Parminder S Suchdev receives salary support from the U.S. Centers for Disease Control & Prevention.
Luz Maria De‐Regil ‐ The Micronutrient Initiative supports the implementation of large‐scale research projects that provide multiple micronutrient powders to children six to 23 months of age as well as programmes that provide iron and folic acid supplements to pregnant women. None of them met the inclusion criteria of this review.
Juan Pablo Peña‐Rosas ‐ The Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development receives financial support from the Government of Luxembourg, the Micronutrient Initiative, US Centers for Disease Control and Prevention and the Bill & Melinda Gates Foundation for its work in several projects.
Figures







Update of
- doi: 10.1002/14651858.CD011158
References
References to studies included in this review
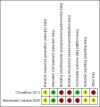
Choudhury 2012 {published data only}
-
- Choudhury N, Aimone A, Hyder SM, Zlotkin SH. Relative efficacy of micronutrient powders versus iron‐folic acid tablets in controlling anemia in women in the second trimester of pregnancy. Food & Nutrition Bulletin 2012;33(2):142‐9. - PubMed
Hernández Cabrera 2008 {published and unpublished data}
-
- Colchero A, Neufeld LM. Cost estimations of different types of micronutrient supplements for children and pregnant women. FASEB Journal 2008;22:Abstract No:678.21.
-
- Hernández Cabrera A, García Guerra A, Domínguez CP, García Feregrino R, Neufeld LM. Effect of three supplements with identical micronutrient content on anemia in pregnant Mexican women. FASEB Journal 2008;22:Abstract No: 677.8.
-
- Neufeld L. Efficacy of 3 nutritional supplements to improve diverse outcomes in children under 2 years of age and pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 19 July 2013] 2007.
-
- Young SL, Blanco I, Hernandez‐Cordero S, Pelto GH, Neufeld LM. Organoleptic properties, ease of use, and perceived health effects are determinants of acceptability of micronutrient supplements among poor Mexican women. Journal of Nutrition 2010;140(3):605‐11. - PubMed
References to studies excluded from this review
Hartman‐Craven 2009 {published data only}
Khambalia 2009 {published data only}
-
- Khambalia A. Periconceptional iron supplementation and iron and folate status among pregnant and non‐pregnant women in rural Bangladesh: PhD thesis. Toronto: University of Toronto, 2009.
-
- Khambalia A, O'Connor D, Zlotkin S. Reduced anemia and improved iron and folate status before and after pregnancy among females in rural Bangladesh is related to length of and adherence to periconceptional iron and folic acid supplementation. FASEB Journal 2009;23 Suppl:917.6.
-
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. American Journal of Clinical Nutrition 2009;90(5):1295‐302. - PubMed
Rim 2014 {unpublished data only}
-
- Rim HY. Use of microencapsulated iron sprinkles to control IDA in pregnant/lactating women and infants in DPR Korea. Institute of Child Nutrition, DPR Korea 2014.
-
- Ritchie S. First Steps Canada (personal communication) 2014.
References to ongoing studies
Richter 2013 {published data only}
-
- Leroy JL. Preventing malnutrition in children under two years of age approach. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 14 June 2014] 2010.
-
- Richter SM, Leroy JL, Olney D, Quinones E, Ruel M. Strengthening and Evaluating the Preventing Malnutrition in Children under 2 Approach in Guatemala: Report of the Enrollment Survey. Washington DC: FHI 360, 2013.
Additional references
Balshem 2010
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Baxter 2014
-
- Baxter J, Roth D, Al Mahmud A, Ahmed T, Islam M, Zlotkin SH. Tablets are preferred and more acceptable than powdered prenatal calcium supplements among pregnant women in Dhaka, Bangladesh. Journal of Nutrition 2014;144(7):1106‐12. - PubMed
Beard 2000
-
- Beard J. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition 2000;71(5 Suppl):1288S‐1294S. - PubMed
Bhutta 2009
-
- Bhutta ZA, Rizvi A, Raza F, Hotwani S, Zaidi S, Moazzam Hossain S, et al. A comparative evaluation of multiple micronutrient and iron‐folic acid supplementation during pregnancy in Pakistan: impact on pregnancy outcomes. Food and Nutrition Bulletin 2009;30(4 Suppl):S496‐S505. - PubMed
Black 2001
-
- Black RE. Micronutrients in pregnancy. British Journal of Nutrition 2001;85(Suppl 2):S193‐S197. - PubMed
Black 2008
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. - PubMed
Black 2013
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M, et al. Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 2013;382(9890):427‐51. - PubMed
Borenstein 2009
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Oxford: John Wiley & Sons Inc, 2009.
CDC 1998
-
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morbidity & Mortality Weekly Report 1998;47:1‐29. - PubMed
Checkley 2010
-
- Checkley W, West KP Jr, Wise RA, Baldwin MR, Wu L, LeClerq SC, et al. Maternal vitamin A supplementation and lung function in offspring. New England Journal of Medicine 2010;362(19):1784‐94. - PubMed
CHERG 2013
-
- Murray‐Kolb LE, Chen L, Chen P, Shapiro M, Caulfield L. CHERG iron report: maternal mortality, child mortality, perinatal mortality, child cognition, and estimates of prevalence of anemia due to iron deficiency. cherg.org/publications/iron‐report.pdf (accessed February 5 2014) 2013.
Christian 2000
-
- Christian P, West KP Jr, Khatry SK, Kimbrough‐Pradhan E, LeClerq SC, Katz J, et al. Night blindness during pregnancy and subsequent mortality among women in Nepal: effects of vitamin A and beta‐carotene supplementation. American Journal of Epidemiology 2000;152(6):542‐7. - PubMed
Christian 2010
-
- Christian P. Micronutrients, birth weight, and survival. Annual Review of Nutrition 2010;30:83‐104. - PubMed
Commission of the European Communities 1993
-
- Commission of the European Communities. Nutrient and Energy Intakes for the European Community. Reports of the Scientific Committee for Food. Luxembourg: Commission of the European Communities, 1993.
Dalmiya 2009
-
- Dalmiya D, Darnton‐Hill I, Schultink W, Shrimpton R. Multiple micronutrient supplementation during pregnancy: a decade of collaboration in action. Food and Nutrition Bulletin 2009;30(4 Suppl):S477‐479. - PubMed
de Pee 2007
-
- Pee S, Moench‐Pfanner R, Martini E, Zlotkin S, Darton‐Hill I, Bloem MW. Home‐fortification in emergency response and transition programming: experiences in Aceh and Nias, Indonesia. Food and Nutrition Bulletin 2007;28:189‐97. - PubMed
de Pee 2008
-
- Pee S, Kraemer K, Briel T, Boy E, Grasset C, Moench‐Pfanner R, et al. Quality criteria for micronutrient powder products. Food and Nutrition Bulletin 2008;29(3):232‐41. - PubMed
De‐Regil 2010
De‐Regil 2011
De‐Regil 2012
Duley 2009
-
- Duley L. The global impact of pre‐eclampsia and eclampsia. Seminars in Perinatology 2009;33(3):130‐7. - PubMed
Fall 2009
Glinoer 2007
-
- Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutrition 2007;10(12A):1542‐6. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Haider 2012
Haws 2007
-
- Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL. Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy and Planning 2007;22(4):193‐215. - PubMed
HFTAG 2013
-
- Home Fortification Technical Advisory Group. A Manual for Developing and Implementing Monitoring Systems for Home Fortification Interventions. Geneva: HFTAG, 2013.
HFTAG 2013b
-
- HFTAG. Home Fortification with Micronutrient Powders (MNP). Basel: Sight and Life, 2013.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huffman 1998
-
- Huffmann SL, Baker J, Schumann J, Zehner ER. The Case for Promoting Multiple Vitamin/Mineral Supplements for Women of Reproductive Age in Developing Countries. Washington: The LINKAGES Project, 1998.
Liu 2013
Liyanage 2002
-
- Liyanage C, Zlotkin S. Bioavailability of iron from micro‐encapsulated iron sprinkle supplement. Food and Nutrition Bulletin 2002;23:133‐7. - PubMed
Muthayya 2013
Nguyen 2008
NICE 2008
-
- National Institute for Health and Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: National Institute for Health and Clinical Excellence, 2008.
O'Neil 2014
-
- O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. Journal of Clinical Epidemiology 2014;67(1):56‐64. - PubMed
Oppenheimer 2001
-
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131:616S‐635S. - PubMed
Peña‐Rosas 2012a
Peña‐Rosas 2012b
Prentice 2007
-
- Prentice AM, Ghattas H, Doherty C, Cox SE. Iron metabolism and malaria. Food and Nutrition Bulletin 2007;28(4):S524‐S539. - PubMed
Raiten 2011
-
- Raiten DJ, Namaste S, Brabin B. Considerations for the safe and effective use of iron interventions in areas of malaria burden ‐ executive summary. International Journal for Vitamin and Nutrition Research 2011;81(1):57‐71. - PubMed
Ray 2007
-
- Ray JG, Wyatt PR, Thompson MD, Vermeulen MJ, Meier C, Wong PY, et al. Vitamin B12 and the risk of neural tube defects in a folic‐acid‐fortified population. Epidemiology 2007;18(3):362‐6. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salam 2013
SGHI 2008
-
- Sprinkles Global Health Initiative (SGHI). Micronutrient sprinkles for use in infants and young children: guidelines on recommendations for use and program monitoring and evaluation. http://www.sghi.org/resource_centre/GuidelinesGen2008.pdf (Accessed September 10 2010) 2008.
Shah 2009
Shaheen 2014
Shankar 2008
-
- Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, et al. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double‐blind cluster‐randomised trial. Lancet 2008;371(9608):215‐27. - PubMed
Shrimpton 2009
-
- Shrimpton R, Huffman S, Zehner ER, Darnton‐Hill I, Dalmiya N. Multiple micronutrient supplementation during pregnancy in developing‐country settings: Policy and program implications of the results of a meta‐analysis. Food and Nutrition Bulletin 2009;30(4):S556‐S573. - PubMed
Soofi 2013
-
- Soofi S, Cousens S, Iqbal SP, Akhund T, Bhutta Z. Impact of provision of daily zinc and iron with multiple micronutrients on growth and morbidity among young children in Pakistan: a cluster RCT of micronutrient powders in Pakistan. Lancet 2013;382(9886):29‐40. - PubMed
Stevens 2013
-
- Stevens GA, Finucane MM, De‐Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: a systematic analysis of population‐representative data. Lancet Global Health 2013;1(1):e16‐e25. - PMC - PubMed
Suchdev 2011
-
- Suchdev PS, De‐Regil LM, Walleser S, Vist GE, Peña‐Rosas JP. Multiple micronutrient powders for home (point of use) fortification of foods in pregnant women: a systematic review. WHO e‐Library of Evidence for Nutrition Actions (http://apps.who.int/iris/bitstream/10665/44748/1/9789241502559_eng.pdf) [accessed September 2014] 2011.
Suchdev 2013
-
- Suchdev PS, Neufeld LM. Micronutrient powders for young children. Lancet 2013;382(9899):1171. - PubMed
Tamura 2006
-
- Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition 2006;83:993‐1016. - PubMed
The Micronutrient Initiative 2009
-
- The Micronutrient Initiative (MI) Flour Fortification Initiative, USAID, GAIN, WHO, The World Bank, UNICEF. Investing in the Future: a United Call to Action on Vitamin and Mineral Deficiencies: Global Report 2009. Ottawa: The Micronutrient Initiative, 2009.
Thorne‐Lyman 2012
Tielsch 2008
-
- Tielsch JM, Rahmathullah L, Katz J, Thulasiraj RD, Coles C, Sheeladevi S, et al. Maternal night blindness during pregnancy Is associated with low birthweight, morbidity, and poor growth in South India. Journal of Nutrition 2008;138(4):787‐92. - PubMed
Titaley 2010
-
- Titaley CR, Dibley MJ, Roberts CL, Agho K. Combined iron/folic acid supplements and malaria prophylaxis reduce neonatal mortality in 19 sub‐Saharan African countries. American Journal of Clinical Nutrition 2010;92(1):235‐43. - PubMed
UNHCR 2011
-
- UNHCR. UNHCR Operational Guidance on the Use of Special Nutritional Products to Reduce Micronutrient Deficiencies and Malnutrition in Refugee Populations. Geneva: UNHCR, 2011.
UNICEF 2004a
-
- UNICEF. Multiple micronutrient supplements to enhance foetal and infant survival, growth and development. Workshop to Review Effectiveness Trials; 2004 15‐18 June; Bangkok, Thailand. 2004.
UNICEF 2004b
-
- UNICEF. Multiple micronutrient supplementation during pregnancy (MMSDP): a review of progress of efficacy trials. UNICEF/UNU/WHO Study Team. Report of a Meeting Held 2004 21‐23 June; Bangkok, Thailand. Centre for International Child Health, Institute of Child Health, University College London and UNICEF, 2004.
UNICEF/CDC 2014
-
- UNICEF, CDC. Global assessment of home fortification interventions, 2011. Home Fortification Technical Advisory Group. Geneva: Home Fortification Technical Advisory Group, 2014.
UNICEF/WHO/UNU 1999
-
- UNICEF/WHO/UNU. Composition of a multi‐micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. Report of an UNICEF/WHO/UNU workshop (http://www.idpas.org/pdf/059CompositionofMult‐MicronutrientSupplement.pdf). New York: UNICEF, 1999.
van den Broek 2010
Villar 1997
-
- Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica 1997;76(1):1‐14. - PubMed
WFP 2013
-
- World Food Programme. WFP Strategic Plan (2014–2017). WFP/EB.A/2013/5‐A/1, 2013.
WHO 1994
-
- World Health Organization. Iodine and Health: a Statement by the World Health Organization. WHO/NUT/94.4. Geneva: World Health Organization, 1994.
WHO 2008
-
- World Health Organization. The International Pharmacopoeia. (http://apps.who.int/phint/en/p/about/) (accessed 2008) 2008.
WHO 2009a
-
- World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. http://www.who.int/healthinfo/global_burden_disease/ GlobalHealthRisks_report_full.pdf (accessed July 2011) 2009:1‐62.
WHO 2009b
-
- World Health Organization (WHO). Global Prevalence of Vitamin A Deficiency in Populations at risk 1995‐2005: WHO global Database on Vitamin A Deficiency. Geneva: World Health Organization, 2009.
WHO 2010
-
- World Health Organization. Malaria. In: Poumerol G, Wilder‐Smith A editor(s). Malaria. International Travel and Health. Situation as on 1 January 2010. Geneva: World Health Organization, 2010.
WHO 2011a
-
- World Health Organization. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Pregnant Women. Geneva: World Health Organization, 2011. - PubMed
WHO 2011b
-
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplements for Pregnant Women. Geneva: World Health Organization, 2011.
WHO/FAO 2006
-
- World Health Organization/Food and Agriculture Organization of the United Nations. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations, 2006.
Zeng 2009
-
- Zeng L, Yan H, Cheng Y, Dang S, Dibley MJ. Adherence and costs of micronutrient supplementation in pregnancy in a double‐blind, randomized, controlled trial in rural western China. Food and Nutrition Bulletin 2009;30(4 Suppl):S480‐S487. - PubMed
Zimmermann 2008
-
- Zimmermann MB, Jooste PL, Pandav CS. Iodine‐deficiency disorders. Lancet 2008;372(9645):1251‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

