The long subclinical phase of Mycobacterium avium ssp. paratuberculosis infections explained without adaptive immunity
- PMID: 26092036
- PMCID: PMC4473850
- DOI: 10.1186/s13567-015-0202-3
The long subclinical phase of Mycobacterium avium ssp. paratuberculosis infections explained without adaptive immunity
Abstract
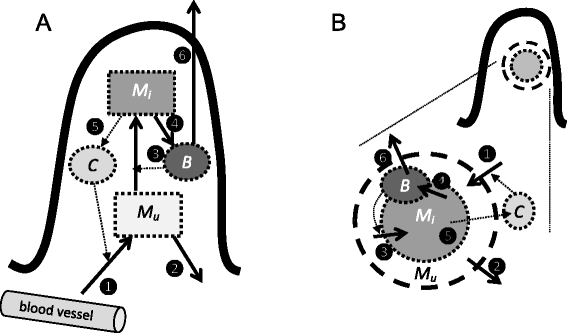
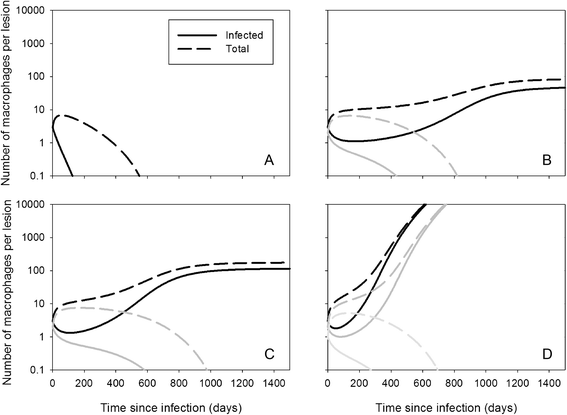
Mycobacterium avium ssp. paratuberculosis (MAP) is an infection of the ruminant intestine. In cows, a long subclinical phase with no or low intermittent shedding precedes the clinical phase with high shedding. It is generally considered that an adaptive cell-mediated immune response controls the infection during the subclinical phase, followed by unprotective antibodies later in life. Based on recent observations, we challenge the importance of adaptive immunity and instead suggest a role of the structural organization of infected macrophages in localized granulomatous lesions. We investigated this hypothesis by mathematical modelling. Our first model describes infection in a villus, assuming a constant lesion volume. This model shows the existence of two threshold parameters, the MAP reproduction ratio R MAP determining if a lesion can develop, and the macrophage replacement ratio R MF determining if recruitment of macrophages is sufficient for unlimited growth. We show that changes in R MF during a cow's life - i.e. changes in the innate immune response - can cause intermittent shedding. Our second model describes infection in a granuloma, assuming a growing lesion volume. This model confirms the results of the villus model, and can explain early slow granuloma development: small granulomas grow slower because bacteria leave the granuloma quickly through the relatively large surface area. In conclusion, our models show that the long subclinical period of MAP infection can result from the structural organization of the infection in granulomatous lesions with an important role for innate rather than adaptive immunity. It thus provides a reasonable hypothesis that needs further investigation.
Figures



References
-
- Mitchell RM, Medley GF, Collins MT, Schukken YH. A meta-analysis of the effect of dose and age at exposure on shedding of Mycobacterium avium subspecies paratuberculosis (MAP) in experimentally infected calves and cows. Epidemiol Infect. 2012;140:231–246. doi: 10.1017/S0950268811000689. - DOI - PubMed
-
- Larsen AB, Merkal RS, Cutlip RC. Age of cattle as related to resistance to infection with Mycobactierium paratuberculosis. Am J Vet Res. 1975;36:255–257. - PubMed
-
- Sweeney RW, Jones DE, Habecker P, Scott P. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res. 1998;59:842–847. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

