FRAX and the effect of teriparatide on vertebral and non-vertebral fracture
- PMID: 26092063
- PMCID: PMC4913866
- DOI: 10.1007/s00198-015-3173-3
FRAX and the effect of teriparatide on vertebral and non-vertebral fracture
Abstract
Daily teriparatide injections have been shown to reduce vertebral and non-vertebral fractures. Here, we demonstrate that the magnitude of fracture risk reduction is independent of baseline fracture probability assessed by FRAX.
Introduction: Daily administration of 20 or 40 μg teriparatide has been shown to significantly decrease the risk of vertebral and non-vertebral fracture compared with placebo. The aim of the present study was to evaluate fracture risk assessed at baseline using the FRAX® tool and to determine the efficacy of teriparatide as a function of baseline fracture risk.
Methods: One thousand six hundred thirty-seven postmenopausal women in the pivotal phase three trial, randomly assigned to receive placebo (n = 544), teriparatide 20 μg per day (n = 541) or teriparatide 40 μg per day (n = 552), were studied. Baseline clinical risk factors were entered into country-specific FRAX models to compute the 10-year probability of major osteoporotic fractures with or without input of femoral neck BMD. Because there was no difference in effect of 20 and 40 μg teriparatide daily on fracture occurrence, the two active groups were merged. The interaction between probability of a major fracture and treatment efficacy was examined by Poisson regression.
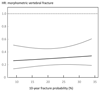
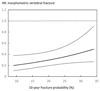
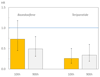
Results: The 10-year probability of major osteoporotic fractures (with BMD) ranged from 2.2-67.2 %. Treatment with teriparatide was associated with a 37 % decrease in all non-vertebral fractures (95 % CI 10-56 %) and a 56 % decrease in low-energy non-vertebral fractures (95 % CI 24-75 %) compared with placebo. The risk of morphometric vertebral fractures decreased significantly by 66 % (95 % CI 50-77 %). Hazard ratios for the effect of teriparatide on the fracture outcome did not change significantly with increasing fracture probability (p > 0.30). Similar findings were noted for the interaction when BMD was excluded from the FRAX model, or when probability of hip fracture was used as the marker of baseline risk.
Conclusion: We conclude that teriparatide significantly decreases the risk of non-vertebral and morphometric vertebral fractures in women by a similar extent, irrespective of baseline fracture probability.
Keywords: BMD; Epidemiology; FRAX; Fracture; Interaction; Osteoporosis; Randomised controlled trial; Teriparatide.
Conflict of interest statement
NH has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma. JA Kanis has received consulting fees, advisory board fees, lecture fees, and/or grant support from the majority of companies concerned with skeletal metabolism. EVM has received consultancy, lecture fees, research grant support and/or honoraria from ActiveSignal, Alliance for Better Bone Health, AMGEN, Bayer, Consilient Healthcare, GE Lunar, Hologic, Internis Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Servier, Tethys, UCB and Univadis. RTB and BHM are employees and shareholders of Eli Lilly and Company.
Figures

References
-
- Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International. 2013;24:23–57. - PMC - PubMed
-
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. - PubMed
-
- Prince R, Sipos A, Hossain A, et al. Sustained non vertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20:1507–1513. - PubMed
-
- Kanis JA, on behalf of the World Health Organization Scientific Group . Assessment of osteoporosis at the primary health-care level Technical Report. WHO Collaborating Centre, University of Sheffield; UK: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

