Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity
- PMID: 26092137
- PMCID: PMC7154219
- DOI: 10.1002/14651858.CD002250.pub3
Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity
Abstract
Background: Several studies have suggested that prophylactic antibiotics given during pregnancy improved maternal and perinatal outcomes, while others have shown no benefit and some have reported adverse effects.
Objectives: To determine the effect of prophylactic antibiotics on maternal and perinatal outcomes during the second and third trimester of pregnancy for all women or women at risk of preterm delivery.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2015) and reference lists of retrieved articles.
Selection criteria: Randomised controlled trials comparing prophylactic antibiotic treatment with placebo or no treatment for women in the second or third trimester of pregnancy before labour.
Data collection and analysis: We assessed trial quality and extracted data.
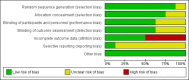
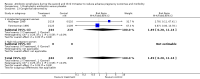
Main results: The review included eight randomised controlled trials. Approximately 4300 women were recruited to detect the effect of prophylactic antibiotic administration on pregnancy outcomes. Primary outcomesAntibiotic prophylaxis did not reduce the risk of preterm prelabour rupture of membranes (risk ratio (RR) 0.31; 95% confidence interval (CI) 0.06 to 1.49 (one trial, 229 women), low quality evidence) or preterm delivery (RR 0.88; 95% CI 0.72 to 1.09 (six trials, 3663 women), highquality evidence). However, preterm delivery was reduced in the subgroup of pregnant women with a previous preterm birth who had bacterial vaginosis (BV) during the current pregnancy (RR 0.64; 95% CI 0.47 to 0.88 (one trial, 258 women)), but there was no reduction in the subgroup of pregnant women with previous preterm birth without BV during the pregnancy (RR 1.08; 95% CI 0.66 to 1.77 (two trials, 500 women)). A reduction in the risk of postpartum endometritis (RR 0.55; 95% CI 0.33 to 0.92 (one trial, 196 women)) was observed in high-risk pregnant women (women with a history of preterm birth, low birthweight, stillbirth or early perinatal death) and in all women (RR 0.53; 95% CI 0.35 to 0.82 (three trials, 627 women), moderate quality evidence). There was no difference in low birthweight (RR 0.86; 95% CI 0.53 to 1.39 (four trials; 978 women)) or neonatal sepsis (RR 11.31; 95% CI 0.64 to 200.79) (one trial, 142 women)); and blood culture confirming sepsis was not reported in any of the studies. Secondary outcomesAntibiotic prophylaxis reduced the risk of prelabour rupture of membranes (RR 0.34; 95% CI 0.15 to 0.78 (one trial, 229 women), low quality evidence) and gonococcal infection (RR 0.35; 95% CI 0.13 to 0.94 (one trial, 204 women)). There were no differences observed in other secondary outcomes (congenital abnormality; small-for-gestational age; perinatal mortality), whilst many other secondary outcomes (e.g. intrapartum fever needing treatment with antibiotics) were not reported in included trials.Regarding the route of antibiotic administration, vaginal antibiotic prophylaxis during pregnancy did not prevent infectious pregnancy outcomes. The overall risk of bias was low, except that incomplete outcome data produced high risk of bias in some studies. The quality of the evidence using GRADE was assessed as low for preterm prelabour rupture of membranes, high for preterm delivery, moderate for postpartum endometritis, low for prelabour rupture of membranes, and very low for chorioamnionitis. Intrapartum fever needing treatment with antibiotics was not reported in any of the included studies.
Authors' conclusions: Antibiotic prophylaxis did not reduce the risk of preterm prelabour rupture of membranes or preterm delivery (apart from in the subgroup of women with a previous preterm birth who had bacterial vaginosis). Antibiotic prophylaxis given during the second or third trimester of pregnancy reduced the risk of postpartum endometritis, term pregnancy with pre-labour rupture of membranes and gonococcal infection when given routinely to all pregnant women. Substantial bias possibly exists in the review's results because of a high rate of loss to follow-up and the small numbers of studies included in each of our analyses. There is also insufficient evidence on possible harmful effects on the baby. Therefore, we conclude that there is not enough evidence to support the use of routine antibiotics during pregnancy to prevent infectious adverse effects on pregnancy outcomes.
Conflict of interest statement
None known.
Figures















Update of
-
Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity.Cochrane Database Syst Rev. 2015 Jan 26;1:CD002250. doi: 10.1002/14651858.CD002250.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2015 Jun 20;(6):CD002250. doi: 10.1002/14651858.CD002250.pub3. PMID: 25621770 Updated.
References
References to studies included in this review
Gichangi 1997 {published data only}
-
- Gichangi PB, Ndinya‐Achola JO, Ombete J, Nagelkerke NJ, Temmerman M. Antimicrobial prophylaxis in pregnancy: a randomized placebo‐controlled trial with cefetamet‐pivoxil in pregnant women with a poor obstetric history. American Journal of Obstetrics and Gynecology 1997;177:680‐4. - PubMed
Hauth 1995 {published data only}
-
- Hauth JC, Goldenberg LR, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. New England Journal of Medicine 1995;333:1732‐6. - PubMed
McGregor 1990 {published data only}
-
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Franco‐Buff A, et al. Prospective, double‐blinded, randomized, placebo‐controlled trial of short‐course erythromycin (E) base in women at high risk for preterm birth. Proceedings of the Society for Gynecologic Investigation; 1988. 1988.
-
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double‐blind, placebo‐controlled trial of erythromycin treatment. American Journal of Obstetrics and Gynecology 1990;163:1580‐91. - PubMed
Paul 1997 {published data only}
-
- Paul VK, Singh M, Buckshee K. Erythromycin treatment of pregnant women to reduce the incidence of low birth weight and preterm deliveries. International Journal of Gynecology & Obstetrics 1998;62:87‐8. - PubMed
Sen 2005 {published data only}
-
- Sen A, Mahalanabis D, Mukhopadhyay S, Chakrabarty K, Singh AK, Bisai S, et al. Routine use of antimicrobials by pregnant Indian women does not improve birth outcome: a randomized controlled trial. Journal of Health, Population & Nutrition 2005;23(3):236‐44. - PubMed
Temmerman 1995 {published data only}
-
- Temmerman M, Njagi E, Nagelkerke N, Ndinya‐Achola J, Plummer FA, Meheus A. Mass antimicrobial treatment in pregnancy, a randomized, placebo‐controlled trial in a population with high rates of sexually transmitted diseases. Journal of Reproductive Medicine 1995;40:176‐80. - PubMed
Van den Broek 2009 {published data only}
Vermeulen 1999 {published data only}
-
- Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomised placebo‐controlled double‐blind trial. British Journal of Obstetrics and Gynaecology 1999;106:652‐7. - PubMed
References to studies excluded from this review
Andrews 2003 {published data only}
-
- Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin‐positive women. Obstetrics & Gynecology 2003;101(5 Pt 1):847‐55. - PubMed
-
- Hendler I, Andrews WW, Carey CJ, Klebanoff MA, Noble WD, Sibai BM, et al. The relationship between resolution of asymptomatic bacterial vaginosis and spontaneous preterm birth in fetal fibronectin‐positive women. American Journal of Obstetrics and Gynecology 2007;197(5):488.e1‐488.e5. - PubMed
-
- Lin M, for the NICHD MFMU Network. Impact of quantitative fetal fibronectin (FFN) change of efficacy of antibiotic vs. placebo treatment of asymptomatic FFN positive women to prevent subsequent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S56.
-
- Ramsey P. Relationship of midtrimester fetal fibronectin (FFN) concentration to antibiotic efficacy for the prevention of spontaneous preterm birth in asymptomatic FFN positive women [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S11.
Andrews 2006 {published data only}
-
- Andrews W, Goldenberg R, Hauth J, Cliver S. Interconceptional antibiotics to prevent spontaneous preterm birth (SPTB): a randomized trial [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):s57. - PubMed
-
- Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2006;194(3):617‐23. - PubMed
-
- Tita A, Cliver S, Goepfert A, Goldenberg R, Conner M, Andrews W. Impact of interconceptional antibiotics on the "endometrial flora". American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S17. - PubMed
-
- Tita A, Cliver S, Goepfert A, Goldenberg R, Conner M, Andrews W. Interconceptional antibiotics and preterm delivery: subgroup analyses. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S76. - PubMed
Audebert 1989 {published data only}
-
- Audebert AJM. Use of polygynax in pregnant women for preventing cervico‐vaginal infections. Revue Francaise de Gynecologie et d Obstetrique 1989;84:287‐90. - PubMed
Goldenberg 2006 {published data only}
-
- Goldenberg R, Andrews W, Taha T, Fawzi W, Brown E. HIVNET 024: fetal fibronectin (FFN), preterm birth (PTB) and mother to child transmission of HIV (MTCT) in African women with HIV [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S52.
-
- Goldenberg R, Mudenda V, Brown E, Taha T, Kaaya E. HIVNET 024: neutrophilic and mononuclear placental infiltration, antibiotics and outcome in an African population [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S188.
-
- Goldenberg R, Taha T, Hoffman I, Fawzi W, Mwatha A. HPTN 024: antibiotics do not prevent preterm birth (PTB) in an HIV‐infected African population [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S3.
-
- Goldenberg RL, Mudenda V, Read JS, Brown ER, Sinkala M, Kamiza S, et al. HPTN 024 study: histologic chorioamnionitis, antibiotics and adverse infant outcomes in a predominantly HIV‐1‐infected African population. American Journal of Obstetrics & Gynecology 2006;195(4):1065‐74. - PubMed
Gray 2001 {published data only}
-
- Gray RH, Wabwire‐Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. American Journal of Obstetrics & Gynecology 2001;185:1209‐17. - PubMed
-
- Kigozi GG, Brahmbhatt H, Wabwire‐Mangen F, Wawer MJ, Serwadda D, Sewankambo N, et al. Treatment of trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. American Journal of Obstetrics and Gynecology 2003;189:1398‐400. - PubMed
Larsson 2006 {published data only}
-
- Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U, the premature study group of the southeast health care region of Sweden. Late miscarriage and preterm birth after treatment with clindamycin: a randomised consent design study according to Zelen. BJOG: an international journal of obstetrics and gynaecology 2006;113(6):629‐37. - PubMed
Luntamo 2010 {published data only}
-
- Ashorn P. Gestational sulfadoxine‐pyrimethamine and azithromycin treatment to prevent preterm birth (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
-
- Luntamo M, Kulmala T, Cheung YB, Maleta K, Ashorn P. The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Tropical Medicine & International Health 2013;18(4):386‐97. - PubMed
-
- Luntamo M, Kulmala T, Mbewe B, Cheung YB, Maleta K, Ashorn P. Effect of repeated treatment of pregnant women with sulfadoxine‐ pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2010;83(6):1212‐20. - PMC - PubMed
Peters 1995 {published data only}
Shennan 2006 {published data only}
-
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Minimal quantitative levels of fetal fibronectin (1‐49 NG/ML) at 24‐27 weeks are associated with an increased risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S47. - PubMed
-
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Quantitative fetal fibronectin screening at 24 weeks substantially discriminates the risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. - PubMed
-
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET study. BJOG: an international journal of obstetrics and gynaecology 2006;113(1):65‐74. - PubMed
-
- Thompson C. Taken before their time. Nursing Times 2002;98(3):29.
Tripathi 2008 {published data only}
-
- Gupta S, Tripathi R, Singh N, Bhalla P, Ramji S, Mala YM. Pregnancy outcome in asymptomatic women with abnormal vaginal flora without any treatment and after treatment with vaginal clindamycin and clotrimazole: a randomised controlled trial. South African Journal of Obstetrics and Gynaecology 2013;19(2):35‐8.
-
- Tripathi R, Gupta S, Bhalla P, Mala YM, Tyagi S, Ramji S. Maternal and fetal outcome in patients with abnormal vaginal flora after treatment with vaginal clindamycin in early pregnancy. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):161.
Unger 2015 {published data only}
-
- Rathjen P. Intermittent preventive treatment with azithromycin‐containing regimens in pregnant women in Papua New Guinea (IPTp in PNG). ClinicalTrials.gov (http://clinicaltrials.gov) [accessed April 2013) 2010.
References to ongoing studies
Hoffman 2013 {published data only}
-
- Hoffman M. Clindamycin to reduce preterm birth in a low resource setting. ClinicalTrials.gov (accessed 21 May 2013) 2013.
Additional references
Ashorn 2006
-
- Ashorn P. Gestational sulfadoxine‐pyrimethamine and azithromycin treatment to prevent preterm birth (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
Braun 1971
-
- Braun P, Lee Y, Klein JO, Marcy M, Klein TA, Charles D. Birth weight and genital mycoplasma in pregnancy. New England Journal of Medicine 1971;284:167‐74. - PubMed
Cunningham 1997
-
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC, Hankins GDV, et al. Williams Obstetrics. 20th Edition. Norwalk, CT: Appleton & Lange, 1997.
Eschenbach 1991
-
- Eschenbach DA, Nugent RP, Rao VA, Cotch MF, Gibbs RS, Lipscomb KA, et al. A randomized placebo controlled trial of erythromycin for the treatment of ureaplasma urealyticum in preventing premature delivery. American Journal of Obstetrics and Gynecology 1991;164:734‐42. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Gravett 1986
-
- Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 1986;256:1899‐905. - PubMed
Hardy 1984
-
- Hardy PH, Nell EE, Spence MR, Hardy JB, Graham DA, Rosenbaum RC. Prevalence of six sexually transmitted disease agents among pregnant innercity adolescents and pregnancy outcome. Lancet 1984;8:333‐7. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillier 1995
-
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of low‐birth weight infant. New England Journal of Medicine 1995;333:1737‐42. - PubMed
Kenyon 2008
-
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet 2008;372(9646):1319‐27. - PubMed
Kurtzman 2008
-
- Kurtzman J, Chandiramani M, Briley A, Poston L, Shennan A. Quantitative fetal fibronectin screening at 24 weeks substantially discriminates the risk of recurrent preterm delivery in asymptomatic patients with prior preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S10. - PubMed
Lettieri 1993
-
- Lettieri L, Vintzileos AM, Rodis JF, Albini SM, Saladia CM. Does idiopathic preterm labor resulting in preterm birth exist?. American Journal of Obstetrics and Gynecology 1993;168:1480‐5. - PubMed
Lin 2005
-
- Lin M, for the NICHD MFMU Network. Impact of quantitative fetal fibronectin (FFN) change of efficacy of antibiotic vs. placebo treatment of asymptomatic FFN positive women to prevent subsequent preterm birth [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S56.
McCormack 1987
-
- McCormack WM, Rosner B, Lee YH, Munoz A, Kass CD. Effect on birth weight of erythromycin in treatment of pregnant women. Obstetrics & Gynecology 1987;69:202‐7. - PubMed
Morales 1994
-
- Morales JW, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo controlled double blind study. Obstetrics & Gynecology 1994;171:345‐9. - PubMed
Newton 1989
-
- Newton ER, Dinsmoor MJ, Gibbs RS. A randomised blinded placebo controlled trial of antibiotics in idiopathic preterm labour. Obstetrics & Gynecology 1989;74:562‐6. - PubMed
Oleszczuk 2000
-
- Oleszczuk JJ, Keith LG. Vaginal infection: prophylaxis and perinatal outcome‐a review of the literature. International Journal of Fertility 2000;45(6):358‐67. - PubMed
Rathjen 2010
-
- Rathjen P. Intermittent preventive treatment with azithromycin‐containing regimens in pregnant women in Papua New Guinea (IPTp in PNG). ClinicalTrials.gov (http://clinicaltrials.gov) [accessed April 2013) 2010.
Regan 1981
-
- Regan JA, Chao S, James SL. Premature rupture of membranes, preterm delivery, and group B streptococcal colonization of mothers. American Journal of Obstetrics and Gynecology 1981;141:184‐6. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 1988
-
- Romero R, Mazor M. Infection and preterm labor. Clinical Obstetrics and Gynecology 1988;31:553‐83. - PubMed
Romero 1993
-
- Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, et al. Antibiotic treatment of preterm labour with intact membranes: a multicenter randomised double blinded controlled trial. American Journal of Obstetrics and Gynecology 1993;169:764‐74. - PubMed
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. - PubMed
References to other published versions of this review
Thinkhamrop 2002
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

