Melanin-concentrating hormone is necessary for olanzapine-inhibited locomotor activity in male mice
- PMID: 26092201
- PMCID: PMC4609648
- DOI: 10.1016/j.euroneuro.2015.05.010
Melanin-concentrating hormone is necessary for olanzapine-inhibited locomotor activity in male mice
Abstract
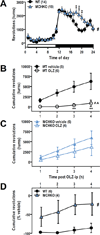
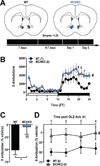
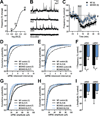
Olanzapine (OLZ), an atypical antipsychotic, can be effective in treating patients with restricting type anorexia nervosa who exercise excessively. Clinical improvements include weight gain and reduced pathological hyperactivity. However the neuronal populations and mechanisms underlying OLZ actions are not known. We studied the effects of OLZ on hyperactivity using male mice lacking the hypothalamic neuropeptide melanin-concentrating hormone (MCHKO) that are lean and hyperactive. We compared the in vivo effects of systemic or intra-accumbens nucleus (Acb) OLZ administration on locomotor activity in WT and MCHKO littermates. Acute systemic OLZ treatment in WT mice significantly reduced locomotor activity, an effect that is substantially attenuated in MCHKO mice. Furthermore, OLZ infusion directly into the Acb of WT mice reduced locomotor activity, but not in MCHKO mice. To identify contributing neuronal mechanisms, we assessed the effect of OLZ treatment on Acb synaptic transmission ex vivo and in vitro. Intraperitoneal OLZ treatment reduced Acb GABAergic activity in WT but not MCHKO neurons. This effect was also seen in vitro by applying OLZ to acute brain slices. OLZ reduced the frequency and amplitude of GABAergic activity that was more robust in WT than MCHKO Acb. These findings indicate that OLZ reduced Acb GABAergic transmission and that MCH is necessary for the hypolocomotor effects of OLZ.
Keywords: Accumbens nucleus; Anorexia; Antipsychotic; Electrophysiology; Locomotion; MCH.
Copyright © 2015 Elsevier B.V. and ECNP. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
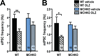
References
-
- Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15:139–150. - PubMed
-
- Brewerton TD. Antipsychotic agents in the treatment of anorexia nervosa: neuropsychopharmacologic rationale and evidence from controlled trials. Curr Psychiatry Rep. 2012;14:398–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

