ROBIS: A new tool to assess risk of bias in systematic reviews was developed
- PMID: 26092286
- PMCID: PMC4687950
- DOI: 10.1016/j.jclinepi.2015.06.005
ROBIS: A new tool to assess risk of bias in systematic reviews was developed
Abstract
Objective: To develop ROBIS, a new tool for assessing the risk of bias in systematic reviews (rather than in primary studies).
Study design and setting: We used four-stage approach to develop ROBIS: define the scope, review the evidence base, hold a face-to-face meeting, and refine the tool through piloting.
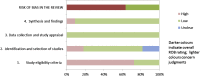
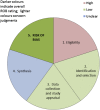
Results: ROBIS is currently aimed at four broad categories of reviews mainly within health care settings: interventions, diagnosis, prognosis, and etiology. The target audience of ROBIS is primarily guideline developers, authors of overviews of systematic reviews ("reviews of reviews"), and review authors who might want to assess or avoid risk of bias in their reviews. The tool is completed in three phases: (1) assess relevance (optional), (2) identify concerns with the review process, and (3) judge risk of bias. Phase 2 covers four domains through which bias may be introduced into a systematic review: study eligibility criteria; identification and selection of studies; data collection and study appraisal; and synthesis and findings. Phase 3 assesses the overall risk of bias in the interpretation of review findings and whether this considered limitations identified in any of the phase 2 domains. Signaling questions are included to help judge concerns with the review process (phase 2) and the overall risk of bias in the review (phase 3); these questions flag aspects of review design related to the potential for bias and aim to help assessors judge risk of bias in the review process, results, and conclusions.
Conclusions: ROBIS is the first rigorously developed tool designed specifically to assess the risk of bias in systematic reviews.
Keywords: Evidence; Meta-analysis; Quality; Risk of bias; Systematic review; Tool.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
The rationale for rating risk of bias should be fully reported: response.J Clin Epidemiol. 2016 Aug;76:239. doi: 10.1016/j.jclinepi.2016.03.008. Epub 2016 Mar 24. J Clin Epidemiol. 2016. PMID: 27034095 No abstract available.
-
The rationale for rating risk of bias should be fully reported.J Clin Epidemiol. 2016 Aug;76:238. doi: 10.1016/j.jclinepi.2016.03.007. Epub 2016 Mar 24. J Clin Epidemiol. 2016. PMID: 27034096 No abstract available.
References
-
- Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org/ Available at. Accessed March 23, 2011.
-
- Centre for Reviews and Dissemination . University of York; York: 2009. Systematic Reviews: CRD's guidance for undertaking reviews in health care [Internet]http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm Available at. Accessed March 23, 2011.
-
- Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E., editors. Clinical practice guidelines we can trust. National Academies Press (US); Washington (DC): 2011. - PubMed
-
- Chandler J., Churchill R., Higgins J., Lasserson T., Tovey D. The Cochrane Collaboration; 2012. Methodological Expectations of Cochrane Intervention Reviews (MECIR): methodological standard for the conduct of new Cochrane Intervention Reviews.http://www.editorial-unit.cochrane.org/mecir Version 2.2. Available at. Accessed 17 December 2012.
-
- Eden J., Levit L., Berg A.O., Morton S., editors. Finding what works in health care: standards for systematic reviews. The National Academies Press; Washington, D.C: 2011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

