α-Melanocyte-stimulating-hormone (α-MSH) modulates human chondrocyte activation induced by proinflammatory cytokines
- PMID: 26093672
- PMCID: PMC4475285
- DOI: 10.1186/s12891-015-0615-1
α-Melanocyte-stimulating-hormone (α-MSH) modulates human chondrocyte activation induced by proinflammatory cytokines
Abstract
Background: Alpha-melanocyte-stimulating-hormone (α-MSH) has marked anti-inflammatory potential. Proinflammatory cytokines are critical mediators of the disturbed cartilage homeostasis in osteoarthritis, inhibiting anabolic activities and increasing catabolic activities in chondrocytes. Since human chondrocytes express α-MSH receptors, we evaluated the role of the peptide in modulating chondrocyte production of pro-inflammatory cytokines, matrix metalloproteinases (MMPs), tissue inhibitors of MMPs (TIMPs), inducible nitric oxide synthase (iNOS) and nitric oxide (NO) in response to interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).
Methods: Human articular chondrocytes were obtained from osteoarthritic joint cartilage from subjects undergoing hip routine arthroplasty procedures. The cells were cultured with or without α-MSH in the presence of IL-1β or TNF-α. Cell-free supernatants were collected and cells immediately lysed for RNA purification. Expression of cytokines, MMPs, TIMPs, iNOS was determined by Reverse Transcription Real-time Polymerase Chain Reaction and enzyme-linked immunosorbent assay. Griess reaction was used for NO quantification.
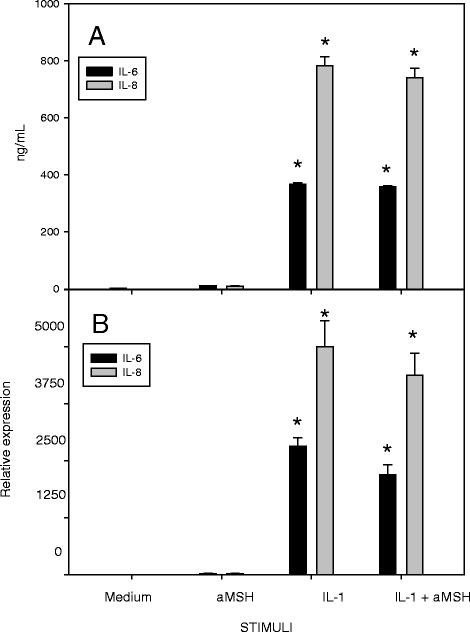
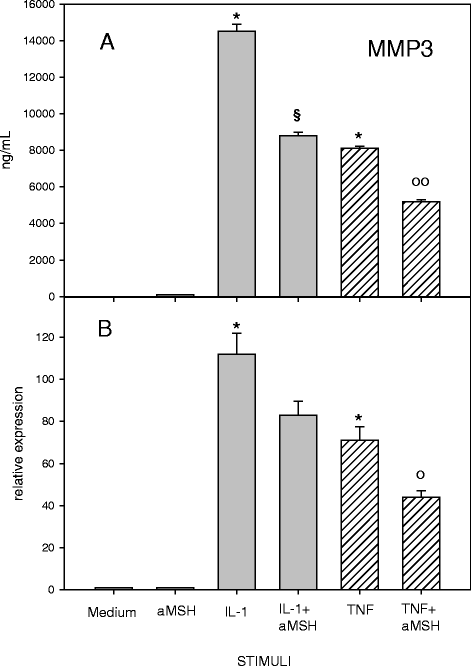
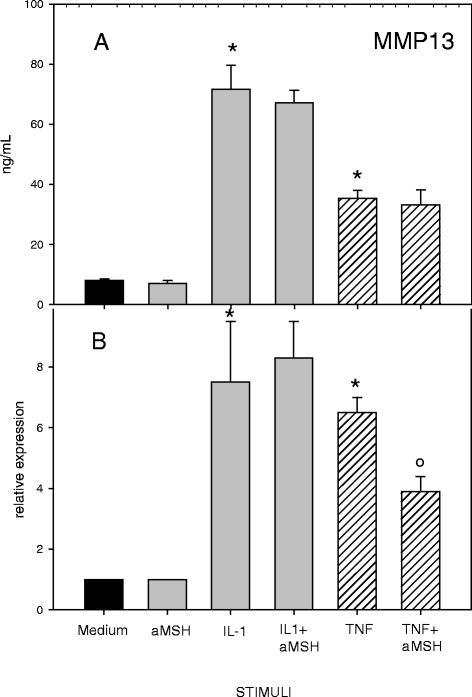
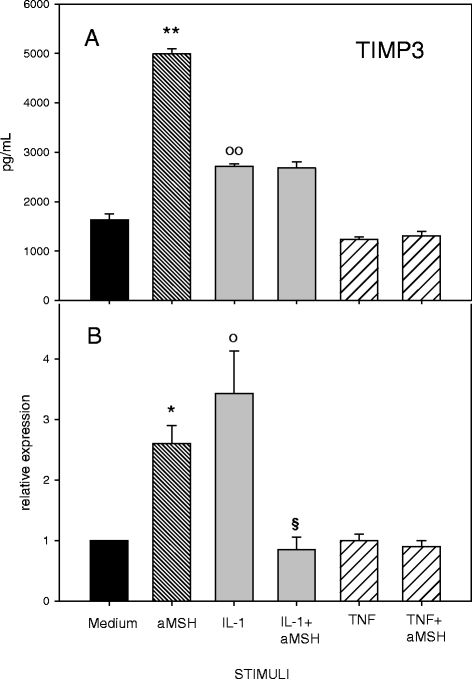
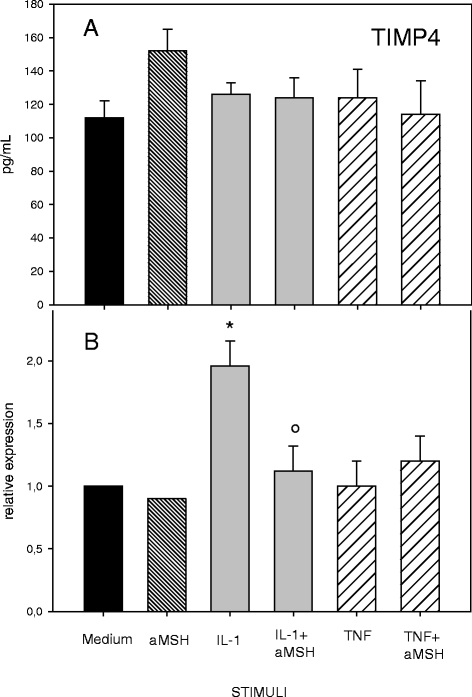
Results: Gene expression and secretion of IL-6, IL-8, MMP-3, MMP-13 were significantly increased in IL-1β or TNF-α-stimulated chondrocytes; α-MSH did not modify the release of IL-6 or IL-8 while the peptide significantly reduced their gene expression on TNF-α-stimulated cells. A significant inhibition of MMP3 gene expression and secretion from IL-1β or TNFα-stimulated chondrocytes was induced by α-MSH. On the other hand, α-MSH did not modify the release of MMP-13 by cytokine-stimulated chondrocyte but significantly decreased gene expression of the molecule on TNF-α-stimulated cells. Detectable amount of TIMP-3 and TIMP-4 were present in the supernatants of resting chondrocytes and a significant increase of TIMP-3 gene expression and release was induced by α-MSH on unstimulated cells. TIMP-3 secretion and gene expression were significantly increased in IL-1β-stimulated chondrocytes and α-MSH down-regulated gene expression but not secretion of the molecule. TIMP-4 gene expression (but not secretion) was moderately induced in IL-1β-stimulated chondrocytes with a down-regulation exerted by α-MSH. IL-1β and TNF-α were potent stimuli for NO production and iNOS gene expression by chondrocytes; no inhibition was induced by α-MSH on cytokine-stimulated NO production, while the peptide significantly reduced gene expression of iNOS.
Conclusions: Our results underscore a potential anti-inflammatory and chondroprotective activity exerted by α-MSH, increasing TIMP-3 gene expression and release on resting cells and down- modulating TNF-α-induced activation of human chondrocytes. However, the discrepancy between the influences exerted by α-MSH on gene expression and protein release as well as the difference in the inhibitory pattern exerted by α-MSH in TNF-α- or IL-1β-stimulated cells leave some uncertainty on the role of the peptide on chondrocyte modulation.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

