RNASEH1 Mutations Impair mtDNA Replication and Cause Adult-Onset Mitochondrial Encephalomyopathy
- PMID: 26094573
- PMCID: PMC4572567
- DOI: 10.1016/j.ajhg.2015.05.013
RNASEH1 Mutations Impair mtDNA Replication and Cause Adult-Onset Mitochondrial Encephalomyopathy
Abstract
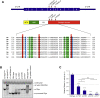
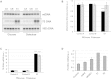
Chronic progressive external ophthalmoplegia (CPEO) is common in mitochondrial disorders and is frequently associated with multiple mtDNA deletions. The onset is typically in adulthood, and affected subjects can also present with general muscle weakness. The underlying genetic defects comprise autosomal-dominant or recessive mutations in several nuclear genes, most of which play a role in mtDNA replication. Next-generation sequencing led to the identification of compound-heterozygous RNASEH1 mutations in two singleton subjects and a homozygous mutation in four siblings. RNASEH1, encoding ribonuclease H1 (RNase H1), is an endonuclease that is present in both the nucleus and mitochondria and digests the RNA component of RNA-DNA hybrids. Unlike mitochondria, the nucleus harbors a second ribonuclease (RNase H2). All affected individuals first presented with CPEO and exercise intolerance in their twenties, and these were followed by muscle weakness, dysphagia, and spino-cerebellar signs with impaired gait coordination, dysmetria, and dysarthria. Ragged-red and cytochrome c oxidase (COX)-negative fibers, together with impaired activity of various mitochondrial respiratory chain complexes, were observed in muscle biopsies of affected subjects. Western blot analysis showed the virtual absence of RNase H1 in total lysate from mutant fibroblasts. By an in vitro assay, we demonstrated that altered RNase H1 has a reduced capability to remove the RNA from RNA-DNA hybrids, confirming their pathogenic role. Given that an increasing amount of evidence indicates the presence of RNA primers during mtDNA replication, this result might also explain the accumulation of mtDNA deletions and underscores the importance of RNase H1 for mtDNA maintenance.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Cerritelli S.M., Crouch R.J. Cloning, expression, and mapping of ribonucleases H of human and mouse related to bacterial RNase HI. Genomics. 1998;53:300–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

