Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions
- PMID: 26095048
- PMCID: PMC4542150
- DOI: 10.1016/j.stem.2015.05.003
Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions
Abstract
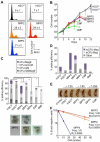
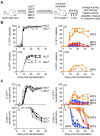
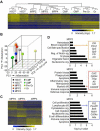
Despite great advances in understanding the mechanisms underlying blood production, lineage specification at the level of multipotent progenitors (MPPs) remains poorly understood. Here, we show that MPP2 and MPP3 are distinct myeloid-biased MPP subsets that work together with lymphoid-primed MPP4 cells to control blood production. We find that all MPPs are produced in parallel by hematopoietic stem cells (HSCs), but with different kinetics and at variable levels depending on hematopoietic demands. We also show that the normally rare myeloid-biased MPPs are transiently overproduced by HSCs in regenerating conditions, hence supporting myeloid amplification to rebuild the hematopoietic system. This shift is accompanied by a reduction in self-renewal activity in regenerating HSCs and reprogramming of MPP4 fate toward the myeloid lineage. Our results support a dynamic model of blood development in which HSCs convey lineage specification through independent production of distinct lineage-biased MPP subsets that, in turn, support lineage expansion and differentiation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. - PubMed
-
- Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. - PubMed
-
- Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
- R01 HL092471/HL/NHLBI NIH HHS/United States
- F32 HL106989/HL/NHLBI NIH HHS/United States
- K01 DK098315/DK/NIDDK NIH HHS/United States
- T32 AI007334/AI/NIAID NIH HHS/United States
- R01 GM109031/GM/NIGMS NIH HHS/United States
- 12765/CRUK_/Cancer Research UK/United Kingdom
- K01DK098315/DK/NIDDK NIH HHS/United States
- R01 CA180778/CA/NCI NIH HHS/United States
- MR/M008975/1/MRC_/Medical Research Council/United Kingdom
- F32HL106989/HL/NHLBI NIH HHS/United States
- 97922/WT_/Wellcome Trust/United Kingdom
- MC_PC_12009/MRC_/Medical Research Council/United Kingdom
- R01HL092471/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

