Structural and Functional Analysis of the GADD34:PP1 eIF2α Phosphatase
- PMID: 26095357
- PMCID: PMC4489983
- DOI: 10.1016/j.celrep.2015.05.043
Structural and Functional Analysis of the GADD34:PP1 eIF2α Phosphatase
Abstract
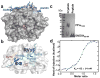
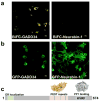
The attenuation of protein synthesis via the phosphorylation of eIF2α is a major stress response of all eukaryotic cells. The growth-arrest- and DNA-damage-induced transcript 34 (GADD34) bound to the serine/threonine protein phosphatase 1 (PP1) is the necessary eIF2α phosphatase complex that returns mammalian cells to normal protein synthesis following stress. The molecular basis by which GADD34 recruits PP1 and its substrate eIF2α are not fully understood, hindering our understanding of the remarkable selectivity of the GADD34:PP1 phosphatase for eIF2α. Here, we report detailed structural and functional analyses of the GADD34:PP1 holoenzyme and its recruitment of eIF2α. The data highlight independent interactions of PP1 and eIF2α with GADD34, demonstrating that GADD34 functions as a scaffold both in vitro and in cells. This work greatly enhances our molecular understanding of a major cellular eIF2α phosphatase and establishes the foundation for future translational work.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. - PubMed
-
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23:1292–1303. - PMC - PubMed
-
- Ceulemans H, Stalmans W, Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. BioEssays: news and reviews in molecular, cellular and developmental biology. 2002;24:371–381. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

