A Cambrian origin for vertebrate rods
- PMID: 26095697
- PMCID: PMC4502669
- DOI: 10.7554/eLife.07166
A Cambrian origin for vertebrate rods
Abstract
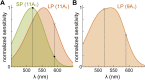
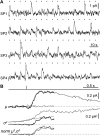
Vertebrates acquired dim-light vision when an ancestral cone evolved into the rod photoreceptor at an unknown stage preceding the last common ancestor of extant jawed vertebrates (~420 million years ago Ma). The jawless lampreys provide a unique opportunity to constrain the timing of this advance, as their line diverged ~505 Ma and later displayed high-morphological stability. We recorded with patch electrodes the inner segment photovoltages and with suction electrodes the outer segment photocurrents of Lampetra fluviatilis retinal photoreceptors. Several key functional features of jawed vertebrate rods are present in their phylogenetically homologous photoreceptors in lamprey: crucially, the efficient amplification of the effect of single photons, measured by multiple parameters, and the flow of rod signals into cones. These results make convergent evolution in the jawless and jawed vertebrate lines unlikely and indicate an early origin of rods, implying strong selective pressure toward dim-light vision in Cambrian ecosystems.
Keywords: evolution; lamprey; neuroscience; photoreceptors; retina; rods.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




References
-
- Arnemo R. Ionic composition of some lakes in central sweden. Oikos. 1964;15:44–55. doi: 10.2307/3564747. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

