Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations
- PMID: 26095797
- PMCID: PMC4476039
- DOI: 10.1038/srep11198
Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations
Abstract
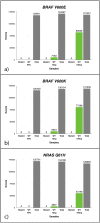
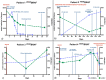
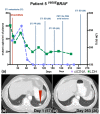
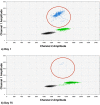
We assessed the utility of droplet digital PCR (ddPCR) to evaluate the potential of using circulating tumour DNA (ctDNA) as a post therapy monitoring tool in melanoma by comparing it to serum LDH levels and RECIST scores. ddPCR was shown to be reliable in distinguishing mutant from wild type alleles with no false positives. Subsequently, we quantified ctDNA ((V600E)BRAF,(V600K)BRAF or (Q61H)NRAS) in 6 stage IV melanoma patients across several time points during their treatment course. All tested patients had detectable ctDNA, which exhibited dynamic changes corresponding to the changes in their disease status. The ctDNA levels fell upon treatment response and rose with detectable disease progression. In our group of patients, ctDNA was more consistent and informative than LDH as a blood-based biomarker. In addition, BRAF mutant ctDNA as detected by ddPCR could be used diagnostically where the tumour block was unavailable. In conclusion, this study demonstrates the applicability of using ddPCR to detect and quantify ctDNA in the plasma of melanoma patients.
Figures

References
-
- Rigel D. S., Russak J. & Friedman R. The Evolution of Melanoma Diagnosis: 25 Years Beyond the ABCDs. CA: A Cancer Journal for Clinicians 60, 301–316 (2010). - PubMed
-
- Australian Institute of Health and Welfare (AIHW) - Australian Government. Cancer in Australia: an overview 2012. Cancer series no. 74. Cat. no. CAN 70. Canberra: AIHW 1–215 (2012).
-
- Eisenhauer E. A. et al.. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 45, 228–247 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

