Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later
- PMID: 26096370
- PMCID: PMC4675706
- DOI: 10.1111/nyas.12794
Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later
Abstract
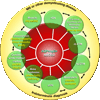
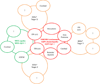
The discovery of AQP4-IgG (a pathogenic antibody that targets the astrocytic water channel aquaporin-4), as the first sensitive and specific biomarker for any inflammatory central nervous system demyelinating disease (IDD), has shifted emphasis from the oligodendrocyte and myelin to the astrocyte as a central immunopathogenic player. Neuromyelitis optica (NMO) spectrum disorders (SDs) represent an evolving spectrum of IDDs extending beyond the optic nerves and spinal cord to include the brain (especially in children) and, rarely, muscle. NMOSD typical brain lesions are located in areas that highly express the target antigen, AQP4, including the circumventricular organs (accounting for intractable nausea and vomiting) and the diencephalon (accounting for sleep disorders, endocrinopathies, and syndrome of inappropriate antidiuresis). Magnetic resonance imaging brain abnormalities fulfill Barkoff criteria for multiple sclerosis in up to 10% of patients. As the spectrum broadens, the importance of highly specific assays that detect pathogenic AQP4-IgG targeting extracellular epitopes of AQP4 cannot be overemphasized. The rapid evolution of our understanding of the immunobiology of AQP4 autoimmunity necessitates continuing revision of NMOSD diagnostic criteria. Here, we describe scientific advances that have occurred since the discovery of NMO-IgG in 2004 and review novel targeted immunotherapies. We also suggest that NMOSDs should now be considered under the umbrella term autoimmune aquaporin-4 channelopathy.
Keywords: aquaporin-4; multiple sclerosis; myelitis; neuromyelitis optica; optic neuritis.
© 2015 New York Academy of Sciences.
Figures

References
-
- Devic E. Myélite aiguë dorso-lombaire avec névrite optique. - Autopsie. Congres francais de medecine. 1895:434–439.
-
- Gault F. De la neuromyelite optique aigue. These: Faculte de Medecine et de Pharmacie. 1894
-
- Jarius S, Wildemann B. An early case of neuromyelitis optica: on a forgotten report by Jacob Lockhart Clarke, FRS. Mult Scler. 2011;17(11):1384–1386. - PubMed
-
- Jarius S, Wildemann B. The case of the Marquis de Causan (1804): an early account of visual loss associated with spinal cord inflammation. J Neurol. 2012;259(7):1354–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

