Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib
- PMID: 26096453
- PMCID: PMC4807634
- DOI: 10.1002/cncr.29493
Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib
Abstract
Background: Epidermal growth factor receptor (EGFR) exon 20 insertions (exon20ins) represent approximately 10% of EGFR-mutant lung adenocarcinomas, and are associated with resistance to EGFR tyrosine kinase inhibitors (TKIs). Clinical outcomes in comparison with patients with sensitizing EGFR mutations are not well established.
Methods: Patients with stage IV lung adenocarcinomas with EGFR exon20ins were identified through routine molecular testing. Clinicopathologic data were collected. Overall survival (OS) was measured from the diagnosis of stage IV disease, and in patients treated with EGFR TKIs, the time to progression (TTP) on erlotinib was measured.
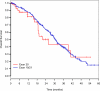
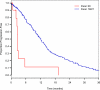
Results: One thousand eight hundred and eighty-two patients with stage IV lung adenocarcinomas were identified: 46 patients had EGFR exon20ins (2%), and 258 patients had an EGFR exon 19 deletion (exon19del)/L858R point mutation (14%). Among 11 patients with lung adenocarcinomas with EGFR exon20ins who received erlotinib, 3 patients (27%) had a partial response (FQEA, 1; ASV, 1; and unknown variant, 1). TTP for patients with EGFR exon20ins and patients with EGFR exon19del/L858R on erlotinib were 3 and 12 months, respectively (P < .01). Responses to chemotherapy were similar for patients with lung adenocarcinomas with EGFR exon20ins and patients with lung adenocarcinomas with EGFR exon19del/L858R. Median OS from the diagnosis of stage IV disease for patients with EGFR exon20ins and patients with EGFR exon19del/L858R was 26 months (95% confidence interval, 19 months-not reached n = 46) and 31 months (95% confidence interval, 28-33 months; n = 258), respectively (P = .53).
Conclusions: The majority of patients with advanced lung adenocarcinomas harboring EGFR exon20ins do not respond to EGFR TKI therapy. Standard chemotherapy should be used as first-line therapy. These patients have an OS similar to that of patients with sensitizing EGFR mutations. Individuals with certain variants such as FQEA and ASV may respond to erlotinib.
Keywords: advanced; epidermal growth factor receptor (EGFR); erlotinib; exon 20 insertion; lung adenocarcinoma.
© 2015 American Cancer Society.
Figures

References
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
-
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

