ClC-1 mutations in myotonia congenita patients: insights into molecular gating mechanisms and genotype-phenotype correlation
- PMID: 26096614
- PMCID: PMC4594292
- DOI: 10.1113/JP270358
ClC-1 mutations in myotonia congenita patients: insights into molecular gating mechanisms and genotype-phenotype correlation
Abstract
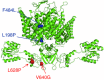
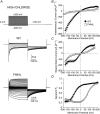
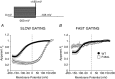
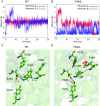
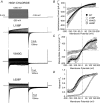
Key points: Loss-of-function mutations of the skeletal muscle ClC-1 channel cause myotonia congenita with variable phenotypes. Using patch clamp we show that F484L, located in the conducting pore, probably induces mild dominant myotonia by right-shifting the slow gating of ClC-1 channel, without exerting a dominant-negative effect on the wild-type (WT) subunit. Molecular dynamics simulations suggest that F484L affects the slow gate by increasing the frequency and the stability of H-bond formation between E232 in helix F and Y578 in helix R. Three other myotonic ClC-1 mutations are shown to produce distinct effects on channel function: L198P shifts the slow gate to positive potentials, V640G reduces channel activity, while L628P displays a WT-like behaviour (electrophysiology data only). Our results provide novel insight into the molecular mechanisms underlying normal and altered ClC-1 function.
Abstract: Myotonia congenita is an inherited disease caused by loss-of-function mutations of the skeletal muscle ClC-1 chloride channel, characterized by impaired muscle relaxation after contraction and stiffness. In the present study, we provided an in-depth characterization of F484L, a mutation previously identified in dominant myotonia, in order to define the genotype-phenotype correlation, and to elucidate the contribution of this pore residue to the mechanisms of ClC-1 gating. Patch-clamp recordings showed that F484L reduced chloride currents at every tested potential and dramatically right-shifted the voltage dependence of slow gating, thus contributing to the mild clinical phenotype of affected heterozygote carriers. Unlike dominant mutations located at the dimer interface, no dominant-negative effect was observed when F484L mutant subunits were co-expressed with wild type. Molecular dynamics simulations further revealed that F484L affected the slow gate by increasing the frequency and stability of the H-bond formation between the pore residue E232 and the R helix residue Y578. In addition, using patch-clamp electrophysiology, we characterized three other myotonic ClC-1 mutations. We proved that the dominant L198P mutation in the channel pore also right-shifted the voltage dependence of slow gating, recapitulating mild myotonia. The recessive V640G mutant drastically reduced channel function, which probably accounts for myotonia. In contrast, the recessive L628P mutant produced currents very similar to wild type, suggesting that the occurrence of the compound truncating mutation (Q812X) or other muscle-specific mechanisms accounted for the severe symptoms observed in this family. Our results provide novel insight into the molecular mechanisms underlying normal and altered ClC-1 function.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures





References
-
- Adelman SA. Doll JD. Generalized Langevin equation approach for atom/solid-surface scattering: General formulation for classical scattering off harmonic solids. J Chem Phys. 2008;64:2375–2388.
-
- Alberga D, Nicolotti O, Lattanzi G, Nicchia GP, Frigeri A, Pisani F, Benfenati V. Mangiatordi GF. A new gating site in human aquaporin-4: Insights from molecular dynamics simulations. Biochim Biophys Acta. 2014;1838:3052–3060. - PubMed
-
- Bennetts B. Parker MW. Molecular determinants of common gating of a ClC chloride channel. Nat Commun. 2013;4:2507–2517. - PubMed
-
- Bennetts B, Parker MW. Cromer BA. Inhibition of skeletal muscle ClC-1 chloride channels by low intracellular pH and ATP. J Biol Chem. 2007;282:32780–32791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

