Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases
- PMID: 26096795
- PMCID: PMC4591179
- DOI: 10.1111/febs.13342
Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases
Abstract
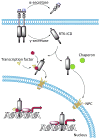
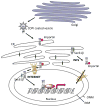
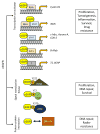
Intracellular localization has been reported for over three-quarters of receptor tyrosine kinase (RTK) families in response to environmental stimuli. Internalized RTK may bind to non-canonical substrates and affect various cellular processes. Many of the intracellular RTKs exist as fragmented forms that are generated by γ-secretase cleavage of the full-length receptor, shedding, alternative splicing, or alternative translation initiation. Soluble RTK fragments are stabilized and intracellularly transported into subcellular compartments, such as the nucleus, by binding to chaperone or transcription factors, while membrane-bound RTKs (full-length or truncated) are transported from the plasma membrane to the ER through the well-established Rab- or clathrin adaptor protein-coated vesicle retrograde trafficking pathways. Subsequent nuclear transport of membrane-bound RTK may occur via two pathways, INFS or INTERNET, with the former characterized by release of receptors from the ER into the cytosol and the latter characterized by release of membrane-bound receptor from the ER into the nucleoplasm through the inner nuclear membrane. Although most non-canonical intracellular RTK signaling is related to transcriptional regulation, there may be other functions that have yet to be discovered. In this review, we summarize the proteolytic processing, intracellular trafficking and nuclear functions of RTKs, and discuss how they promote cancer progression, and their clinical implications.
Keywords: INTERNET; MRIN; RTK cleavage; intracellular trafficking; non-canonical RTK functions; nuclear receptor tyrosine kinases; nuclear translocation; proteolytic cleavage; receptor tyrosine kinase; retrograde trafficking.
© 2015 FEBS.
Figures



References
-
- Keefe DM, Bateman EH. Tumor control versus adverse events with targeted anticancer therapies. Nat Rev Clin Oncol. 2012;9:98–109. - PubMed
-
- Templeton AJ, Diez-Gonzalez L, Ace O, Vera-Badillo F, Seruga B, Jordan J, Amir E, Pandiella A, Ocana A. Prognostic relevance of receptor tyrosine kinase expression in breast cancer: A meta-analysis. Cancer Treat Rev 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

