Intravenous Pharmacokinetics, Local Tolerability, and Hemolysis of an SBE7-β-Cyclodextrin Formulation of the Neurokinin-1 Receptor Antagonist Vestipitant
- PMID: 26097793
- PMCID: PMC4467239
- DOI: 10.1002/cpdd.128
Intravenous Pharmacokinetics, Local Tolerability, and Hemolysis of an SBE7-β-Cyclodextrin Formulation of the Neurokinin-1 Receptor Antagonist Vestipitant
Abstract
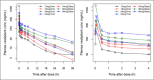
Vestipitant is a potent and selective neurokinin 1 (NK-1) receptor antagonist that was investigated as a potential treatment for post-operative nausea and vomiting (PONV). A previous mannitol-based formulation of vestipitant was associated with hemolytic activity in preclinical studies. In an effort to reduce the hemolytic potential and develop an IV formulation of vestipitant that could be administered more rapidly, an IV formulation containing sulfobutylether-7-beta-cyclodextrin (SBE7-β-CD, Captisol™) was developed and tested in a phase 1 clinical study. This was a randomized, single-blind (subjects and investigator-blinded, sponsor-unblinded), placebo controlled, dose escalation study in healthy subjects in which 7 cohorts of 8 subjects per cohort received SBE7-β-CD -based vestipitant (2 mg/mL) or placebo (saline) in a 3:1 ratio (active:placebo) at different doses and infusion rates. The results demonstrated the ability to infuse up to 48 mg vestipitant in a 2 mg/mL formulation over 30 seconds with no evidence of hemolytic effects. Cohorts of subjects at lower doses and longer infusion duration (>1 minute) reported more AEs related to the infusion site than those at the higher doses and faster infusion rates.
Keywords: NK-1 receptor antagonist; SBE7-β-cyclodextrin; hemolysis; intravenous; vestipitant.
Figures
References
-
- Roberts C, Inamdar A, Koch A, et al. A randomized controlled study comparing the effects of vestipitant or vestipitant and paroxetine combination in subjects with tinnitus. Otolo Neurolog. 2011;32:721–727. - PubMed
-
- Zanone Poma S, Merlo-Pich E, Bettica P, et al. Anxiolytic effects of vestipitant in a sub-group of healthy volunteers known to be sensitive to CO2 challenge. J Psychopharmacol. 2014;28:491–497. - PubMed
-
- Lasseter KC, Gambale J, Bo J, et al. Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects. J Clin Pharmacol. 2007;47:834–840. - PubMed
-
- Smith BP, Vandenhende FR, DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278–1283. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources